Figure 2.
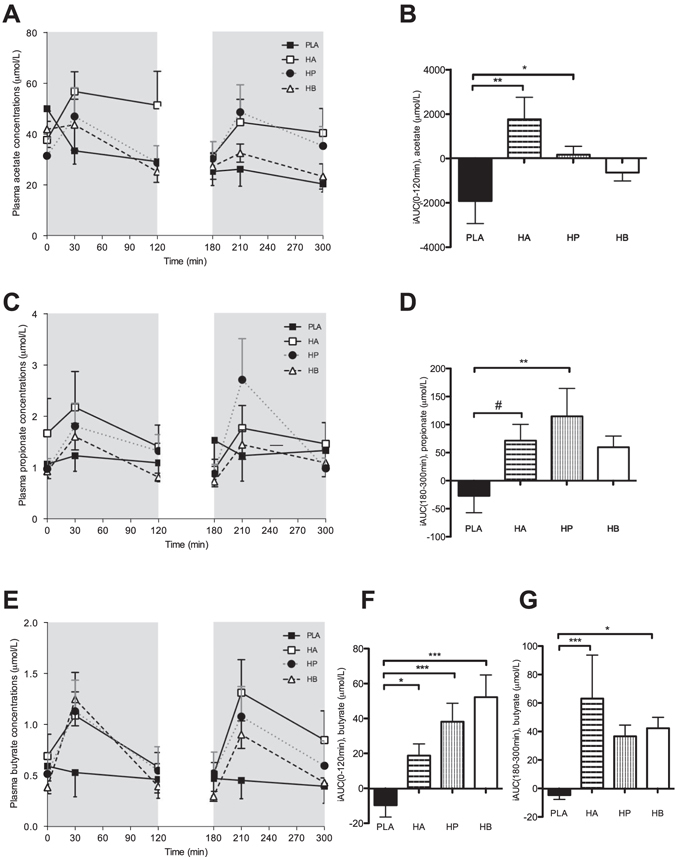
Effect of colonic administration of SCFA mixtures on fasting and postprandial plasma acetate (A,B), propionate (C,D), and butyrate (E–G) concentrations (A) Fasting (t0–t120 min) and postprandial (t180–t300 min) plasma acetate concentrations after colonic SCFA infusions. (B) iAUC for fasting (t0–t120 min) plasma acetate following colonic SCFA infusions. Overall treatment effect for fasting plasma acetate P = 0.051 (period P = 0.338, carry-over P = 0.898). (C) Fasting (t0–t120 min) and postprandial (t180–t300 min) plasma propionate concentrations after colonic SCFA infusions. (D) iAUC for postprandial (t180–t300 min) plasma propionate following colonic SCFA infusions. Overall treatment effect for postprandial plasma propionate P = 0.068 (period P = 0.781, carry-over P = 0.896). (E) Fasting (t0–t120 min) and postprandial (t180–t300 min) plasma butyrate concentrations after colonic SCFA infusions. (F) iAUC for fasting (t0–t120 min) plasma butyrate following colonic SCFA infusions. Overall treatment effect for fasting plasma butyrate P < 0.001 (period P = 0.997, carry-over P = 0.879) (G) iAUC for postprandial (t180–t300 min) plasma butyrate following colonic SCFA infusions. Overall treatment effect for postprandial plasma butyrate P = 0.032 (period P = 0.754, carry-over P = 0.971). Values are means ± SEMs (n = 12). Statistical significance indicated as asterisk (*) when ***P < 0.001, **P < 0.01, *P < 0.05 and as hashtag when # P < 0.10.
