Abstract
The three-dimensional arrangement of chromatin encodes regulatory traits important for nuclear processes such as transcription and replication. Chromatin topology is in part mediated by the architectural protein CCCTC-binding factor (CTCF) that binds to the boundaries of topologically associating domains. Whereas sites of CTCF interactions are well characterized, little is known on how long CTCF binds to chromatin and how binding evolves during the cell cycle. We monitored CTCF-chromatin interactions by live cell single molecule tracking in different phases of the cell cycle. In G1-, S-, and G2-phases, a majority of CTCF molecules was bound transiently (∼0.2 s) to chromatin, whereas minor fractions were bound dynamically (∼4 s) or stably (>15 min). During mitosis, CTCF was mostly excluded from chromatin. Our data suggest that CTCF scans DNA in search for two different subsets of specific target sites and provide information on the timescales over which topologically associating domains might be restructured. During S-phase, dynamic and stable interactions decreased considerably compared to G1-phase, but were resumed in G2-phase, indicating that specific interactions need to be dissolved for replication to proceed.
Main Text
Chromatin exhibits architectural traits ranging from the state of compaction by nucleosomes over DNA loops and topologically associating domains (TADs) to the relative location of whole chromosomes (1, 2). Chromatin topology contributes to the orchestration of vital nuclear tasks such as gene transcription by regulating enhancer and promoter contacts (3) or DNA replication, condensation, and repair (4, 5, 6). A rich variety of biomolecules involved in organizing the genome has been identified, including nuclear lamina, noncoding RNA, and architectural proteins such as cohesin and condensin. Recently, the evolutionary conserved transcription factor CTCF emerged to have fundamental roles in organizing chromatin architecture (7, 8). CTCF functions are associated with gene activation and repression, enhancer-promoter insulation, and separation of chromatin domains, most of which can be attributed to its general role as “master weaver” of chromatin topology (9).
Interphase chromatin is dynamic and exhibits local and long-range movements (10), thereby enabling functional contacts to be initiated and rearranged. For example, rearrangements of the MHC-II locus happen within the first 30 min after upregulation of gene expression by interferon-γ (11). Compatible with dynamic rearrangements, estimates of the dynamical behavior of CTCF and other architectural proteins by fluorescence recovery after photobleaching experiments revealed recovery times on the order of seconds to minutes (12, 13, 14). However, detailed interaction times of architectural proteins with chromatin that would report on their ability to fix chromatin structures over time are missing, leaving questions central to a mechanistic understanding of chromatin organization unanswered.
To observe CTCF in living cells, we created WI-38 cell lines expressing a HaloTag-CTCF fusion protein (Halo-CTCF) from a doxycycline-inducible promoter (Supporting Materials and Methods). 24 h before the experiments, we added 5 ng/μL doxycycline to ensure low Halo-CTCF expression comparable to endogenous CTCF levels as quantified by Western blot and flow cytometry (Fig. S1; Supporting Materials and Methods). Notably, high Halo-CTCF expression led to accumulation of cells in S-phase, consistent with inhibition of cell growth by high CTCF concentrations (Fig. S2) (15). We transiently transfected the cells with RFP-cdt1 and GFP-geminin to distinguish among G1-phase, early S-phase (S-phase), or late S-/G2-phase (G2-phase) (16). Cells in M-phase were identified by staining DNA with Hoechst 33342. We visualized single SiR-labeled Halo-CTCF molecules (SiR-Halo-CTCF) (17) by inclined illumination on a custom-built microscope (Supporting Materials and Methods). Free SiR dye was efficiently removed during wash steps (Movie S1). In G1-, S-, and G2-phases, we could observe diffusing as well as bound SiR-Halo-CTCF molecules in the nucleus, revealing diverse and widespread interaction kinetics of CTCF with chromatin (Fig. S3; Movies S2, S3, and S4). In contrast, CTCF was excluded from chromatin in M-phase, with only a few molecules interacting at various timescales (Fig. S4; Movie S5).
We characterized the binding times and number of interaction states between CTCF and chromatin in G1-, S-, and G2-phases by monitoring the fluorescent on-times of individual SiR-Halo-CTCF molecules using time-lapse illumination with different dark times (Fig. S5; Supporting Materials and Methods) (18). First, we determined the effective rate constant as a function of the time-lapse time by fitting a single-exponential decay model to each on-time histogram (Fig. S6). This approach is very sensitive toward the number of distinct dissociation rate constants of the protein (18) and suggested three interaction states for CTCF (Supporting Materials and Methods). We therefore next determined the values of the dissociation rate constants by globally fitting a triexponential decay model to the fluorescent on-time histograms (Fig. S5; Supporting Materials and Methods). Slow movements of chromatin or cells prevented unambiguous identification of molecules after very long dark times and limited determining the lowest dissociation rate constant. We therefore fixed this rate constant to an upper bound of 10−3/s, as below this value the quality of the fit did not change considerably (Fig. S7).
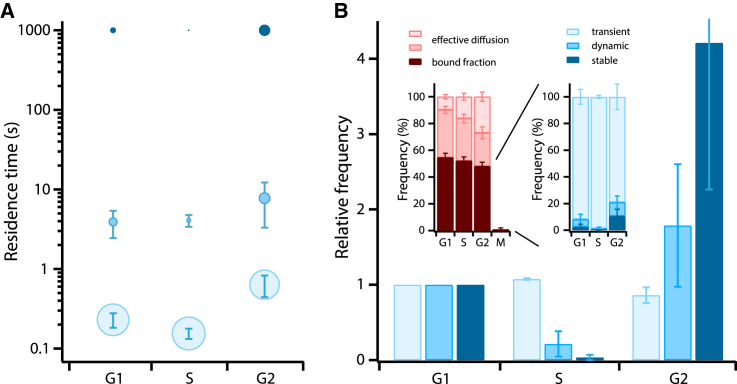
Overall, our analysis revealed that interactions between CTCF and chromatin were grouped into three main regimes (Fig. 1 A). A majority (>80%) of CTCF molecules bound transiently to chromatin with a residence time of 0.2–0.6 s, whereas minor fractions bound dynamically with residence times of 4–8 s or were stable for >1000 s, respectively. The binding times and fractions of CTCF molecules at different cell cycle phases are summarized in Table S1. We observed significant differences in the frequencies of CTCF molecules in each of the interaction states (Fig. 1 B). The fraction of stable bound CTCF molecules decreased by more than a factor of 10 from G1- to S-phase, but recovered in G2-phase and exceeded the value in G1-phase by a factor of four. We found a similar, albeit less pronounced change for the dynamically bound fraction of CTCF. In contrast, the transiently bound fraction increased during S-phase and decreased in G2-phase compared to G1-phase.
Figure 1.
Comparison of residence times and frequencies of SiR-Halo-CTCF-chromatin interactions between different cell cycle phases. (A) Shown here are chromatin residence times of SiR-Halo-CTCF in G1-, S-, and G2-phases. The symbol area is proportional to the fraction of molecules exhibiting the corresponding binding time. Error bars denote SD. (B) Shown here are relative frequencies of SiR-Halo-CTCF molecules exhibiting transient (light blue), dynamic (blue), and stable (dark blue) interactions with chromatin in S- and G2-phases compared to G1-phase. (Left inset) Shown here are fractions of molecules bound to chromatin (dark red) and of molecules exhibiting effective diffusion (red and light red). (Right inset) Shown here are fractions of molecules exhibiting transient (light blue), dynamic (blue), and stable (dark blue) interactions with chromatin in G1-, S-, and G2-phases. Error bars denote SD. To see this figure in color, go online.
To characterize whether the overall associations of CTCF to chromatin changed over the course of the cell cycle, we quantified the percentage of all bound SiR-Halo-CTCF molecules by measuring the distribution of step distances of SiR-Halo-CTCF in G1-, S-, and G2-phases (Fig. S8) (18), or by comparing intensities adjacent to and colocalizing with chromatin in M-phase (Supporting Materials and Methods). We observed a small decrease of bound CTCF molecules from G1- to G2-phase and a strong drop in M-phase (Fig. 1 B, left inset and Table S1).
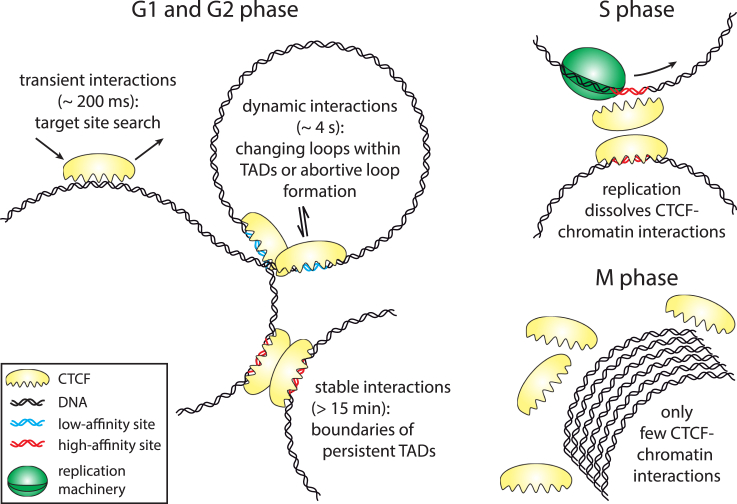
The shortest CTCF-chromatin interaction time is comparable to a transient binding state that has been observed in addition to longer binding events for many DNA binding proteins in single molecule tracking experiments (19, 20, 21, 22, 23). Several independent studies identified the class of transient interactions as interactions with DNA sequences unspecific for the transcription factor (23, 24, 25), whereas longer binding events in the range of seconds were associated with binding of the transcription factor to specific target sites (19, 20, 21, 22, 23). We thus suppose that the major fraction of transiently binding CTCF molecules associate to unspecific DNA sequences for short times in search for specific CTCF target sequences (Fig. 2).
Figure 2.
Shown here is a model of cell-cycle-dependent CTCF-chromatin interactions. To see this figure in color, go online.
The remaining two binding populations of CTCF exhibit binding times in the range of seconds and minutes, pointing to interactions with specific DNA sequences with divergent binding affinity. In the genome, ∼100,000 CTCF binding sites have been identified (26), most of which are located at TAD boundaries (27, 28), but also within TADs (27, 29). CTCF binding sites have been categorized into different classes based on divergent chromatin immune-precipitation occupancy scores (30, 31). This is consistent with differential usage of CTCF zinc finger domains in recognizing different binding sites (14, 32, 33, 34) and mediating orientational binding to different sequences (35). Low and high chromatin immune-precipitation occupancy sites could be identified with CTCF target sites of lower and higher binding affinity in in vitro experiments (36). Whereas low-affinity sites tend to be cell-type specific and are associated with high levels of gene expression, high-affinity sites are more conserved (30, 36, 37), reminiscent of the characteristics of loop structures within TADs and of the embracing TAD, respectively (29, 38). Indeed, a correlation between conserved chromatin domain structures and high CTCF motive affinity was revealed in a comparison of chromatin structures between species (29, 39). The dynamic CTCF-chromatin interactions we observed thus might represent interactions with low-affinity CTCF binding sites within TADs, whereas stable CTCF binding events might constitute interactions with high-affinity sites at TAD boundaries (Fig. 2).
This association is in line with the suggestion that DNA loops within TADs are mobile and changing, whereas the embracing TADs tend to be static and persistent during the cell cycle (1). Our model is also compatible with recently proposed loop extrusion models (40, 41) in which stably occupied TAD boundaries would allow for efficient TAD formation, whereas dynamically bound CTCF would enable changing loop structures in nested TADs. Alternatively, dynamic interactions might reflect abortive loop or TAD formation. Future experiments will be necessary to unambiguously associate the CTCF interaction states to underlying chromatin structures. Nevertheless, within this model, our measurements provide information on the timescales over which the organizational features of chromatin might be restructured.
The timing of replication initiation in S-phase is intimately linked to chromatin topology, as TADs define the borders of early and late replication (42). This suggests that chromatin topology is to some extent maintained during S-phase, consistent with cell cycle-dependent Hi-C experiments (6). Intriguingly, we observed a significant decrease of specific CTCF-chromatin interactions in S- compared to G1-phase. Because overexpression of CTCF, which increases the absolute number of bound molecules, led to accumulation of cells in S-phase, dissolution of stable bound CTCF molecules seems to be necessary for undisturbed progression of the cell into G2-phase. We thus propose that competitive binding of CTCF and the replication machinery dissolves CTCF-chromatin interactions (Fig. 2). An analogous situation occurs in the regulation of splicing where bound CTCF slows down RNA polymerase II (43).
In G2-phase, dynamic and stable CTCF-chromatin interactions were resumed and added up to a fraction of ∼20%. Whether these associations provoke a chromatin architecture comparable to G1-phase is unclear, because chromatin topology significantly changes during the transition from S- to M-phase (6). It is also controversial whether CTCF stays bound during mitosis (44, 45). We found that CTCF was predominantly excluded from chromatin in M-phase, with only a few interacting molecules (Fig. 2).
Further elucidating the functional implications of CTCF-chromatin interaction kinetics and the role of CTCF in mediating chromatin topology during the cell cycle will be important future tasks.
Author Contributions
J.C.M.G. conceived the study. M.R. constructed the single molecule microscope. H.A. and J.C.M.G. designed the experiments. H.A. and C.W. performed the experiments. H.A., M.R., C.W., and J.C.M.G. analyzed data. J.C.M.G. wrote the manuscript with contributions from all authors.
Acknowledgments
We thank Stefanie Weber for cloning the Halo-CTCF construct, Anja Palmer for support with single molecule microscopy, and Kai Johnsson (École Polytechnique Fédérale de Lausanne, Switzerland) for providing SiR dye.
This work was supported by the European Research Council (ERC) under the European Union’s Horizon 2020 Research and Innovation Program (No. 637987 ChromArch to J.C.M.G.), the German Research Foundation (No. GE 2631/1-1 to J.C.M.G.), the German Academic Scholarship Foundation (to M.R.), and the DFG Graduate School of Molecular Medicine at Ulm University (to H.A.).
Editor: Antoine van Oijen.
Footnotes
Supporting Materials and Methods, eight figures, one table, and five movies are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(17)30434-4.
Supporting Citations
References (46, 47, 48) appear in the Supporting Material.
Supporting Material
References
- 1.Gibcus J.H., Dekker J. The hierarchy of the 3D genome. Mol. Cell. 2013;49:773–782. doi: 10.1016/j.molcel.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cremer T., Cremer M. Chromosome territories. Cold Spring Harb. Perspect. Biol. 2010;2 doi: 10.1101/cshperspect.a003889. a003889–a003889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marsman J., Horsfield J.A. Long distance relationships: enhancer-promoter communication and dynamic gene transcription. Bioch. Biophys. Acta. 2012;1819:1217–1227. doi: 10.1016/j.bbagrm.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Smith O.K., Aladjem M.I. Chromatin structure and replication origins: determinants of chromosome replication and nuclear organization. J. Mol. Biol. 2014;426:3330–3341. doi: 10.1016/j.jmb.2014.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Misteli T., Soutoglou E. The emerging role of nuclear architecture in DNA repair and genome maintenance. Nat. Rev. Mol. Cell Biol. 2009;10:243–254. doi: 10.1038/nrm2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naumova N., Imakaev M., Dekker J. Organization of the mitotic chromosome. Science. 2013;342:948–953. doi: 10.1126/science.1236083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohlsson R., Bartkuhn M., Renkawitz R. CTCF shapes chromatin by multiple mechanisms: the impact of 20 years of CTCF research on understanding the workings of chromatin. Chromosoma. 2010;119:351–360. doi: 10.1007/s00412-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ong C.-T., Corces V.G. CTCF: an architectural protein bridging genome topology and function. Nat. Rev. Genet. 2014;15:234–246. doi: 10.1038/nrg3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phillips J.E., Corces V.G. CTCF: master weaver of the genome. Cell. 2009;137:1194–1211. doi: 10.1016/j.cell.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hübner M.R., Spector D.L. Chromatin dynamics. Annu. Rev. Biophys. 2010;39:471–489. doi: 10.1146/annurev.biophys.093008.131348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volpi E.V., Chevret E., Sheer D. Large-scale chromatin organization of the major histocompatibility complex and other regions of human chromosome 6 and its response to interferon in interphase nuclei. J. Cell Sci. 2000;113:1565–1576. doi: 10.1242/jcs.113.9.1565. [DOI] [PubMed] [Google Scholar]
- 12.Gerlich D., Hirota T., Ellenberg J. Condensin I stabilizes chromosomes mechanically through a dynamic interaction in live cells. Curr. Biol. 2006;16:333–344. doi: 10.1016/j.cub.2005.12.040. [DOI] [PubMed] [Google Scholar]
- 13.Gerlich D., Koch B., Ellenberg J. Live-cell imaging reveals a stable cohesin-chromatin interaction after but not before DNA replication. Curr. Biol. 2006;16:1571–1578. doi: 10.1016/j.cub.2006.06.068. [DOI] [PubMed] [Google Scholar]
- 14.Nakahashi H., Kwon K.-R.K., Casellas R. A genome-wide map of CTCF multivalency redefines the CTCF code. Cell Reports. 2013;3:1678–1689. doi: 10.1016/j.celrep.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasko J.E.J., Klenova E.M., Lobanenkov V.V. Cell growth inhibition by the multifunctional multivalent zinc-finger factor CTCF. Cancer Res. 2001;61:6002–6007. [PubMed] [Google Scholar]
- 16.Sakaue-Sawano A., Kurokawa H., Miyawaki A. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell. 2008;132:487–498. doi: 10.1016/j.cell.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 17.Lukinavičius G., Umezawa K., Johnsson K. A near-infrared fluorophore for live-cell super-resolution microscopy of cellular proteins. Nat. Chem. 2013;5:132–139. doi: 10.1038/nchem.1546. [DOI] [PubMed] [Google Scholar]
- 18.Gebhardt J.C.M., Suter D.M., Xie X.S. Single-molecule imaging of transcription factor binding to DNA in live mammalian cells. Nat. Methods. 2013;10:421–426. doi: 10.1038/nmeth.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J., Zhang Z., Liu Z. Single-molecule dynamics of enhanceosome assembly in embryonic stem cells. Cell. 2014;156:1274–1285. doi: 10.1016/j.cell.2014.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groeneweg F.L., van Royen M.E., Schaaf M.J.M. Quantitation of glucocorticoid receptor DNA-binding dynamics by single-molecule microscopy and FRAP. PLoS One. 2014;9:e90532. doi: 10.1371/journal.pone.0090532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knight S.C., Xie L., Tjian R. Dynamics of CRISPR-Cas9 genome interrogation in living cells. Science. 2015;350:823–826. doi: 10.1126/science.aac6572. [DOI] [PubMed] [Google Scholar]
- 22.Sugo N., Morimatsu M., Yamamoto N. Single-molecule imaging reveals dynamics of CREB transcription factor bound to its target sequence. Sci. Rep. 2015;5:10662. doi: 10.1038/srep10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morisaki T., Müller W.G., McNally J.G. Single-molecule analysis of transcription factor binding at transcription sites in live cells. Nat. Commun. 2014;5:4456. doi: 10.1038/ncomms5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caccianini L., Normanno D., Dahan M. Single molecule study of non-specific binding kinetics of LacI in mammalian cells. Faraday Discuss. 2015;184:393–400. doi: 10.1039/c5fd00112a. [DOI] [PubMed] [Google Scholar]
- 25.Ball D.A., Mehta G.D., Karpova T.S. Single molecule tracking of Ace1p in Saccharomyces cerevisiae defines a characteristic residence time for non-specific interactions of transcription factors with chromatin. Nucleic Acids Res. 2016;44:e160. doi: 10.1093/nar/gkw744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim T.H., Abdullaev Z.K., Ren B. Analysis of the vertebrate insulator protein CTCF-binding sites in the human genome. Cell. 2007;128:1231–1245. doi: 10.1016/j.cell.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dixon J.R., Selvaraj S., Ren B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485:376–380. doi: 10.1038/nature11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rao S.S.P., Huntley M.H., Aiden E.L. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159:1665–1680. doi: 10.1016/j.cell.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang Z., Luo O.J., Ruan Y. CTCF-mediated human 3D genome architecture reveals chromatin topology for transcription. Cell. 2015;163:1611–1627. doi: 10.1016/j.cell.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Essien K., Vigneau S., Hannenhalli S. CTCF binding site classes exhibit distinct evolutionary, genomic, epigenomic and transcriptomic features. Genome Biol. 2009;10:R131. doi: 10.1186/gb-2009-10-11-r131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang R., Wang C., Zhang Z. Functional diversity of CTCFs is encoded in their binding motifs. BMC Genomics. 2015;16:649. doi: 10.1186/s12864-015-1824-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Renda M., Baglivo I., Pedone P.V. Critical DNA binding interactions of the insulator protein CTCF: a small number of zinc fingers mediate strong binding, and a single finger-DNA interaction controls binding at imprinted loci. J. Biol. Chem. 2007;282:33336–33345. doi: 10.1074/jbc.M706213200. [DOI] [PubMed] [Google Scholar]
- 33.Filippova G.N., Fagerlie S., Lobanenkov V.V. An exceptionally conserved transcriptional repressor, CTCF, employs different combinations of zinc fingers to bind diverged promoter sequences of avian and mammalian c-myc oncogenes. Mol. Cell. Biol. 1996;16:2802–2813. doi: 10.1128/mcb.16.6.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao T., Wongtrakoongate P., Felsenfeld G. CTCF recruits centromeric protein CENP-E to the pericentromeric/centromeric regions of chromosomes through unusual CTCF-binding sites. Cell Reports. 2015;12:1704–1714. doi: 10.1016/j.celrep.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo Y., Xu Q., Wu Q. CRISPR inversion of CTCF sites alters genome topology and enhancer/promoter function. Cell. 2015;162:900–910. doi: 10.1016/j.cell.2015.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Plasschaert R.N., Vigneau S., Bartolomei M.S. CTCF binding site sequence differences are associated with unique regulatory and functional trends during embryonic stem cell differentiation. Nucleic Acids Res. 2014;42:774–789. doi: 10.1093/nar/gkt910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt D., Schwalie P.C., Odom D.T. Waves of retrotransposon expansion remodel genome organization and CTCF binding in multiple mammalian lineages. Cell. 2012;148:335–348. doi: 10.1016/j.cell.2011.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen Y., Yue F., Ren B. A map of the cis-regulatory sequences in the mouse genome. Nature. 2012;488:116–120. doi: 10.1038/nature11243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vietri Rudan M., Barrington C., Hadjur S. Comparative Hi-C reveals that CTCF underlies evolution of chromosomal domain architecture. Cell Reports. 2015;10:1297–1309. doi: 10.1016/j.celrep.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fudenberg G., Imakaev M., Mirny L.A. Formation of chromosomal domains by loop extrusion. Cell Reports. 2016;15:2038–2049. doi: 10.1016/j.celrep.2016.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanborn A.L., Rao S.S.P., Aiden E.L. Chromatin extrusion explains key features of loop and domain formation in wild-type and engineered genomes. Proc. Natl. Acad. Sci. USA. 2015;112:E6456–E6465. doi: 10.1073/pnas.1518552112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pope B.D., Ryba T., Gilbert D.M. Topologically associating domains are stable units of replication-timing regulation. Nature. 2014;515:402–405. doi: 10.1038/nature13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shukla S., Kavak E., Oberdoerffer S. CTCF-promoted RNA polymerase II pausing links DNA methylation to splicing. Nature. 2011;479:74–79. doi: 10.1038/nature10442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burke L.J., Zhang R., Renkawitz R. CTCF binding and higher order chromatin structure of the H19 locus are maintained in mitotic chromatin. EMBO J. 2005;24:3291–3300. doi: 10.1038/sj.emboj.7600793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zuin J., Franke V., Wendt K.S. A cohesin-independent role for NIPBL at promoters provides insights in CdLS. PLoS Genet. 2014;10:e1004153. doi: 10.1371/journal.pgen.1004153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayflick L., Moorhead P.S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 47.Los G.V., Encell L.P., Wood K.V. HaloTag: a novel protein labeling technology for cell imaging and protein analysis. ACS Chem. Biol. 2008;3:373–382. doi: 10.1021/cb800025k. [DOI] [PubMed] [Google Scholar]
- 48.Efron B., Tibshirani R.J. CRC Press; Boca Raton, FL: 1994. An Introduction to the Bootstrap. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.