ABSTRACT
Improved knowledge regarding the tissue penetration of antituberculosis drugs may help optimize drug management. Patients with drug-resistant pulmonary tuberculosis undergoing adjunctive surgery were enrolled. Serial serum samples were collected, and microdialysis was performed using ex vivo lung tissue to measure pyrazinamide concentrations. Among 10 patients, the median pyrazinamide dose was 24.7 mg/kg of body weight. Imaging revealed predominant lung lesions as cavitary (n = 6 patients), mass-like (n = 3 patients), or consolidative (n = 1 patient). On histopathology examination, all tissue samples had necrosis; eight had a pH of ≤5.5. Tissue samples from two patients were positive for Mycobacterium tuberculosis by culture (pH 5.5 and 7.2). All 10 patients had maximal serum pyrazinamide concentrations within the recommended range of 20 to 60 μg/ml. The median lung tissue free pyrazinamide concentration was 20.96 μg/ml. The median tissue-to-serum pyrazinamide concentration ratio was 0.77 (range, 0.54 to 0.93). There was a significant inverse correlation between tissue pyrazinamide concentrations and the amounts of necrosis (R = −0.66, P = 0.04) and acid-fast bacilli (R = −0.75, P = 0.01) identified by histopathology. We found good penetration of pyrazinamide into lung tissue among patients with pulmonary tuberculosis with a variety of radiological lesion types. Our tissue pH results revealed that most lesions had a pH conducive to pyrazinamide activity. The tissue penetration of pyrazinamide highlights its importance in both drug-susceptible and drug-resistant antituberculosis treatment regimens.
KEYWORDS: tuberculosis, drug resistance, drug penetration, pyrazinamide, microdialysis, pharmacology
INTRODUCTION
While progress has been made in the fight against tuberculosis (TB), the disease continues to be a major public health problem and is now the leading cause of infectious disease-related mortality worldwide (1). Major impediments to improving TB control include inadequate funding, HIV coinfection, and the scourge of drug-resistant Mycobacterium tuberculosis. In 2015, there were an estimated 580,000 patients with rifampin-resistant tuberculosis or multidrug-resistant tuberculosis (MDR TB) and 250,000 MDR TB-related deaths (1). Recently introduced drugs, diagnostics, and global strategic plans have given hope and a way forward to enhancing TB control. The World Health Organization (WHO) recently adopted the End TB Strategy, which provides a long-term plan for combating TB and has a goal of reducing the number of TB deaths by 90% by 2030 (2). The plan is anchored by three pillars of action, including one emphasizing intensified research and innovation. An emerging area of research that may help optimize drug selection and dosing is the evaluation of antituberculosis drug penetration into lung tissue (3).
While M. tuberculosis can invade any organ, TB is predominantly a disease of the lungs, and lesion types and sizes are heterogeneous. The hallmark of progressive pulmonary disease is the cavitary lesion, and this indicator of increased disease severity has been associated with higher rates of acquired drug resistance, treatment failure, and relapse (4–6). A prevailing thought to explain these associations is inadequate drug penetration into tissue. However, currently there are limited data regarding the drug concentrations at the site of disease among patients with pulmonary TB, due in part to the complexities and practicalities in obtaining such measurements. Recent advances in technology and the utilization of innovative methods have made the study of drug penetration into tissue more feasible and have invigorated this area of investigation (7, 8). The majority of recent data evaluating lung tissue antituberculosis drug concentrations has been obtained from animal models (9–11). Further data from humans are needed to better to characterize tissue concentrations in the unique environment and milieu of the TB-diseased human lung.
Our main study aim was to measure the lung tissue concentrations of pyrazinamide among patients with pulmonary TB undergoing adjunctive surgical resection. We chose to study pyrazinamide, given its key role in treating both drug-susceptible and -resistant TB and its ability to preferentially target semidormant bacilli and sterilize lesions. Additional study aims were to evaluate predictors of tissue pyrazinamide concentrations and to measure the pH of resected lesions. Pyrazinamide is converted into its active moiety, pyrazinoic acid (POA), within Mycobacterium tuberculosis, and a low pH outside the mycobacteria favors the accumulation of POA within the mycobacteria, leading to cell death. Thus, lesion pH measurements are essential to understanding the role of prolonged pyrazinamide use in patients with chronic pulmonary disease (12). To evaluate the target site concentrations of pyrazinamide, we utilized the technique of microdialysis (μD), which allows the measurement of unbound (pharmacologically active) extracellular drug concentrations at the site of disease. We have previously shown this method to be successful in measuring antituberculosis drug concentrations among patients with pulmonary TB (13).
(The findings of this study were presented at the 9th International Workshop on Clinical Pharmacology of Tuberculosis Drugs, Liverpool, England, 24 October 2016.)
RESULTS
Study population.
Ten patients undergoing adjunctive surgical resection for drug-resistant TB were enrolled (Table 1). The main indication for surgery (90%) was the presence of a localized lesion along with a high likelihood of relapse on the basis of the patient's clinical status, the level of drug resistance, and radiological lesion appearance. The median age was 30 years; most of the patients were male (80%) and had no history of prior TB treatment (70%). One (10%) patient had diabetes, and two (20%) were coinfected with either hepatitis B or C virus. The median body mass index was 19.5 kg/m2, while the median creatinine clearance was 91.1 ml/min and the albumin level was 4.2 g/dl. Two patients had isoniazid-resistant, rifampin-susceptible TB, while eight had MDR TB (including two with extensively drug-resistant [XDR] TB). The patients had been receiving pyrazinamide prior to surgical resection for a median of 363 days. Eight (80%) patients were receiving a pyrazinamide dose of 1,600 mg per day, and the median dose by weight was 24.7 mg/kg of body weight (range, 22.5 to 33.3 mg/kg).
TABLE 1.
Study population characteristics for 10 patients with drug-resistant pulmonary TB
| Parameter | Value (n = 10) |
|---|---|
| Demographic characteristics | |
| No. (%) of male patients | 8 (80) |
| Median (range) age (yr) | 30.2 (15–54) |
| No. (%) of patients with the following characteristics: | |
| Georgian ethnicitya | 7 (70) |
| Diabetes mellitusb | 1 (10) |
| Hepatitis C antibody positive | 1 (10) |
| Hepatitis B surface antigen positive | 1 (10) |
| Alcohol user | 0 |
| Tobacco user | 3 (30) |
| Retreatment TB case | 3 (30) |
| Median (range) wt (kg) | 53.0 (48–71) |
| Median (range) body mass index (kg/m2) | 19.5 (15–22) |
| Laboratory values | |
| Median (range) creatinine clearancec (ml/min) | 91.1 (52–155) |
| Median (range) albumin level (g/dl) | 4.2 (3.5–4.9) |
| Median (range) hemoglobin level (g/dl) | 13.7 (12.4–15.5) |
| Median (range) alanine aminotransferase level (U/liter) | 18 (10–133) |
| Tuberculosis characteristics and treatment | |
| No. (%) of patient isolates with the following drug susceptibility pattern: | |
| Isoniazid monoresistant | 1 (10) |
| Isoniazid and ofloxacin resistant | 1 (10) |
| MDR | 6 (60) |
| XDR | 2 (20) |
| No. (%) of patients receiving pyrazinamide at a daily dose ofd: | 10 (83) |
| 1,200 mg | 2 (20) |
| 1,600 mg | 8 (80) |
| Median (range) pyrazinamide dose (mg/kg) | 24.7 (22.5–33.3) |
| Median (range) no. of days prior to surgery that the patient had received pyrazinamide | 363 (120–504) |
| No. (%) of patients with the following indication for surgical resection: | |
| Treatment failure and localized lesion | 1 (10) |
| High risk of relapse and localized lesion | 9 (90) |
| No. (%) of patients with the following type of surgery: | |
| Lobectomy | 5 (50) |
| Segmentectomy | 5 (50) |
One patient was Armenian, one was Azeri, and two were of other ethnicities.
The patient was receiving insulin.
Determined using the Cockcroft-Gault equation.
At the time of surgical resection.
Serum pharmacokinetics.
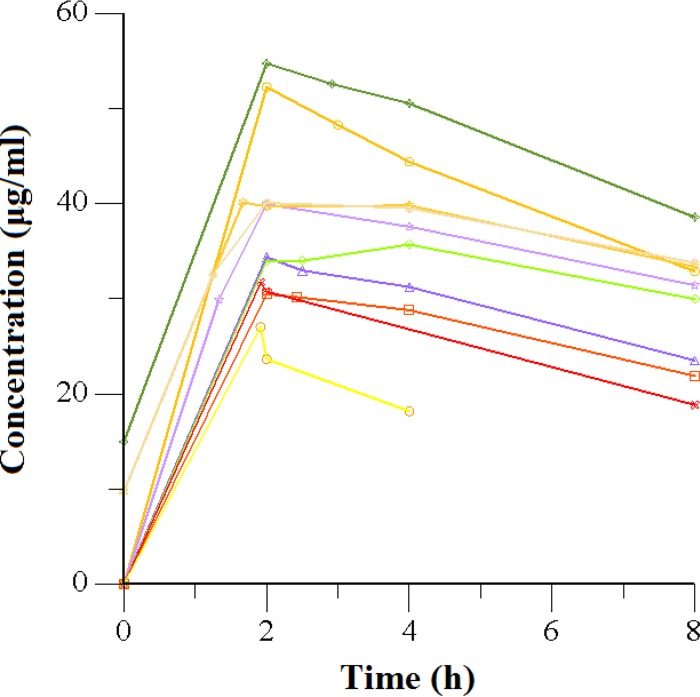
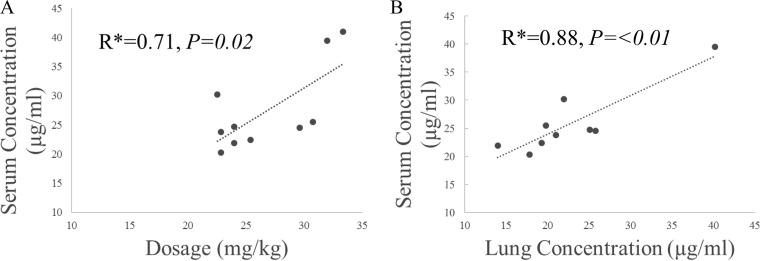
The serum concentration-versus-time graph for pyrazinamide is shown in Fig. 1. All 10 patients (100%) had pyrazinamide maximal serum concentrations (Cmaxs) within the recommended range of 20 to 60 μg/ml. The median half-life (t1/2; 2 h) was similar to values reported in the literature, while the time to Cmax (Tmax; 11.7 h) was slightly higher. There was a significant correlation between the weight-based dosage and Cmax, as shown in Fig. 2A (R = 0.71, P = 0.02). Further results are shown in Table 2.
FIG 1.
Serum concentrations of pyrazinamide versus time after dosing in 10 adults with drug-resistant pulmonary tuberculosis.
FIG 2.
(A) Correlation between peak serum pyrazinamide concentration and dosages; (B) correlation between serum free pyrazinamide concentration and lung tissue pyrazinamide concentration. *, Pearson correlation coefficient.
TABLE 2.
Noncompartmental analysis of serum pyrazinamide concentrations
| Parametera | Median (range) valueb |
|---|---|
| kel (h−1) | 0.059 (0.039–0.13) |
| t1/2 (h) | 11.7 (5.3–17.6) |
| Tmax (h) | 2.0 (1.7–4) |
| Cmax (μg/ml) | 37.8 (27.1–54.7) |
| AUClast (μg · h/ml) | 246.7 (69.6–353.1) |
| AUC0–∞ (μg · h/ml) | 827.6 (208.5–1,139.7) |
| CL/F (liters/h) | 1.9 (1.4–7.7) |
| V/F (liters) | 36.4 (28.6–58.5) |
kel, elimination rate constant; t1/2, half-life; Tmax, time to Cmax; Cmax, maximal serum concentration; AUClast, area under the serum concentration-versus-time curve at the time of the last concentration determination; AUC0–∞, area under the serum concentration-versus-time curve from time zero to infinity; CL, clearance; V, volume of distribution; F, bioavailability (which was assumed to be 1 for purposes of analysis).
Data are for 10 patients receiving pyrazinamide.
Tissue concentrations.
Serum and tissue concentrations were available for 9 of 10 patients receiving pyrazinamide. Data for one patient were excluded due to the collection of an inadequate volume of dialysate. The median lung tissue concentration of free (non-protein-bound) pyrazinamide was 20.96 μg/ml, with the range being 13.95 to 40.17 μg/ml (Table 3). In comparison to the serum free pyrazinamide concentration at the time of surgical resection, the median tissue-to-serum pyrazinamide concentration ratio was 0.77 (range, 0.54 to 0.93). There was a significant correlation between serum and tissue free pyrazinamide concentrations, as shown in Fig. 2B (R = 0.88; P = <0.01).
TABLE 3.
Comparison of free serum and lung tissue pyrazinamide concentrations among patients with drug-resistant pulmonary TB
| Subject | Dose of pyrazinamide (mg/kg) | Serum concn at time of resectiona (μg/ml) | Tissue concn (μg/ml) | Tissue concn/serum concn ratio |
|---|---|---|---|---|
| 1 | 33.33 | 41.04 | NAb | NA |
| 2 | 24 | 28.03 | 25.06 | 0.89 |
| 3 | 24 | 25.67c | 13.95 | 0.54 |
| 4 | 30.77 | 28.91 | 19.78 | 0.68 |
| 5 | 25.40 | 25.44 | 19.29 | 0.76 |
| 6 | 32 | 44.71 | 40.17 | 0.90 |
| 7 | 22.53 | 34.13 | 21.98 | 0.64 |
| 8 | 29.63 | 27.72 | 25.76 | 0.93 |
| 9 | 22.86 | 26.95 | 20.96 | 0.78 |
| 10 | 22.86 | 23.00 | 17.75 | 0.77 |
| Median (range) | 27.87 | 20.96 | 0.77 (0.54–0.93) |
Free serum concentration = measured pyrazinamide concentration × 0.85 (29).
NA, no lung tissue concentration was available for subject 1 due to a low dialysate volume.
For this patient, the serum concentration at the time of surgical resection was estimated with a one-compartment pharmacokinetic model.
Radiology.
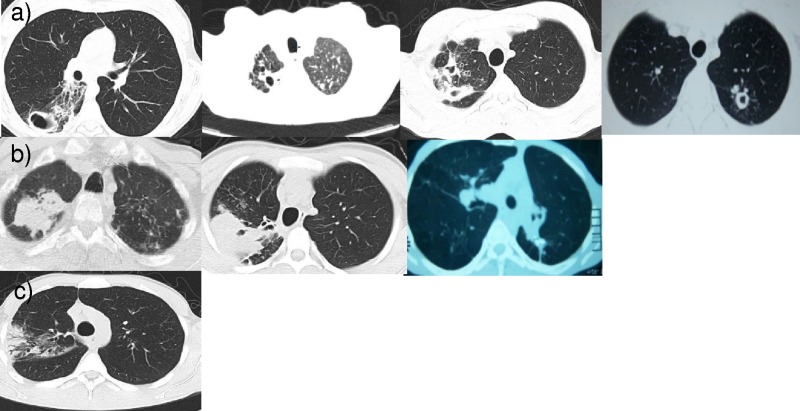
There were seven patients with computed tomography (CT) scans available for reading by study radiologists. For the three patients without preoperative CT scans for review, the radiological reports obtained indicated that all patients had cavitary lesions with a maximum diameter of between 2.1 and 3.5 cm. Among the seven patients with CT scans available for review, mass (n = 3) and cavitary (n = 3) lesions were the most common, and one patient had a consolidation lesion. The majority of patients had pleural thickening (n = 8) and lesions which were connected to an airway (n = 6). Lymphadenopathy was rare (n = 1), and calcifications were present in four patients. A representative CT slice of the predominant lesion for each of the seven patients with films available for review is shown in Fig. 3.
FIG 3.
Representative transverse CT views from the seven patients with films available for review. Three main lesions types were identified, including cavitary lesions (a), mass lesions (b), and a consolidation from one patient (c).
Laboratory results. (i) Pathology.
Pathology examination was performed on all resected tissue specimens, and full results are shown in Table S1 in the supplemental material. Tissue specimens from each patient had granulomas and necrosis present, with most having areas of moderate to severe necrosis (7 of 10, 70%). Tissue specimens for 8 of 10 (80%) patients had a positive acid-fast stain in areas of necrosis. Most tissue specimens demonstrated vascularization (90%) and fibrosis (90%) surrounding the granulomas.
(ii) pH.
The median tissue pH value was 5.5, with the range being 5 to 7.2. Only two tissue samples, including one cavitary lesion and one mass lesion, had a pH of ≥7.0. The two tissue samples with a pH of 7.2 were the only two to have severe necrosis and high numbers of acid-fast-staining organisms on histopathology examination.
(iii) Microbiology and sequencing.
Cultures of tissue specimens from two patients (20%) were positive for M. tuberculosis. Three of five tissue specimens from one patient were positive by culture, while all five tissue specimens from the other patient were positive by culture. The pH values of the tissue specimens from these patients were 5.5 and 7.2. None of the M. tuberculosis isolates had any pncA or rpsA genetic mutations indicating pyrazinamide resistance by whole-genome sequencing.
Correlations with tissue pyrazinamide concentrations and pH.
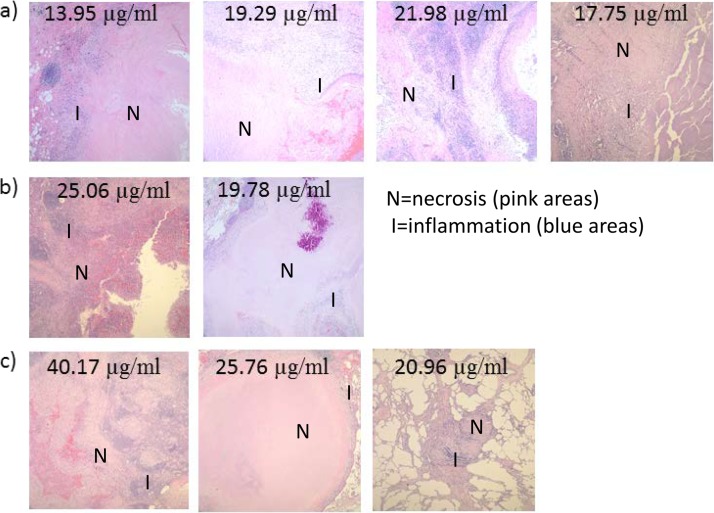
In comparing the association of lesion type and drug tissue penetration, there was no significant difference in the mean free tissue concentrations between cavitary and mass lesions (22.1 and 24.5 μg/ml, respectively; P = 0.71) or the tissue-to-serum pyrazinamide concentration ratios between cavitary and mass lesions (0.78 and 0.73, respectively; P = 0.67). Furthermore, in the seven patients with CT findings for review, no significant differences in tissue pyrazinamide concentrations or tissue concentration-to-serum concentration ratios for lesions with and without calcifications or lesions open or not open to an airway were seen. In correlating the pathology findings with drug tissue penetration, there was a significant negative correlation with tissue free pyrazinamide concentrations and increasing amounts of necrosis (R = −0.66, P = 0.04) and acid-fast-staining organisms (R = −0.75, P = 0.01), as quantified on pathology examination. A representative photomicrograph of tissue stained with hematoxylin and eosin displaying the level of necrosis in the resected lesions of the nine patients for whom a tissue pyrazinamide concentration was available is shown in Fig. 4. There was a similar trend of a negative correlation between the ratio of tissue-to-serum pyrazinamide concentrations and the amounts of necrosis and acid-fast-staining organisms; however, the association was nonsignificant (data not shown). In comparing tissue samples with a pH of ≤5.5 and those with a pH of ≥7.0, there was an association of a higher pH with the amount of acid-fast-staining organisms (P = 0.01) but no association of a higher pH with the amount of necrosis or inflammation, as measured by the amount of polymorphonuclear leukocytes.
FIG 4.
Representative photomicrographs of hematoxylin and eosin-stained specimens for each of the nine patients with tissue pyrazinamide concentrations (listed) available. (a) Cases were classified as severe necrosis when, in addition to what is presented in the photomicrograph, confluent necrosis was present in multiple fields in multiple blocks (two or three blocks). (b) Cases were classified as moderate necrosis when necrosis was confluent with one field. (c) Cases were classified as having rare necrosis when necrosis was scattered within a field. Magnifications, ×4.
DISCUSSION
Among a cohort of chronic TB patients undergoing adjunctive surgical resection, we found good penetration of pyrazinamide into TB-diseased pulmonary tissue, including in cavitary, mass, and consolidation types of lesions. All patients had a tissue concentration-to-serum concentration ratio of ≥0.54 (range, 0.54 to 0.93), and there was a significant correlation of serum and tissue pyrazinamide concentrations, indicating that optimization of serum concentrations should correspondingly optimize lung tissue concentrations. Additionally, we report the first lung tissue pH measurements among TB patients in over 50 years. Our finding of an acidic pH in the large majority (80%) of chronic lesions provides reassuring evidence of an environment conducive to the activity of pyrazinamide. Our findings of drug tissue penetration and acidic pH measurements for tissue support the use of pyrazinamide among patients with pulmonary TB and highlight its importance in both drug-susceptible and multidrug-resistant antituberculosis treatment regimens.
Our study results demonstrating that pyrazinamide penetrates well into various lesion types among patients with pulmonary TB are reassuring, given the importance of pyrazinamide in targeting dormant M. tuberculosis organisms and, hence, being a key sterilizing agent. The range of tissue-to-serum free pyrazinamide concentration ratios was narrow, with most patients having a penetration ratio close to the median value of 0.77, indicating that good tissue penetration can likely be expected in most patients. It is also worth highlighting that our results were for patients with chronic pulmonary TB disease who had lesions characterized histopathologically by fibrosis and necrosis, lesions expected to be harder for drugs to penetrate. Prideaux et al. recently published the only other study to date evaluating the lung tissue penetration of pyrazinamide among human patients (14). Utilizing tissue homogenate drug concentrations, they found an average caseum/plasma pyrazinamide ratio of approximately 0.50, with all patients having a ratio of less than 1, as in our study. While their results were similar to our results, the slight difference in the tissue penetration ratio may have been due to differences in technique, as we compared free unbound pyrazinamide concentrations, whereas the previous investigators compared total pyrazinamide (protein-bound and unbound, extracellular and intracellular) concentrations (14). Prideaux et al. also used a matrix-assisted laser desorption ionization (MALDI) mass spectrometry imaging method to demonstrate a homogeneous distribution of pyrazinamide and its active metabolite, pyrazinoic acid, throughout the lesions, including the cavitary wall, caseum, and cellular components of lesions. These findings complement our study results, which focused on measuring the amount of free pyrazinamide in the center of diseased lung lesions and highlight the pervasive spread of pyrazinamide throughout TB lung lesions.
Our findings revealed an inverse association between tissue pyrazinamide concentrations and increasing amounts of tissue necrosis and acid-fast-staining organisms on histopathology examination. To our knowledge, this is the first time that this relationship has been demonstrated, and while the causal pathway of the association is unclear, it suggests either that pyrazinamide has lower penetration into more severe lung lesions, as characterized by necrosis and the bacillus burden, or that lower penetration may be associated with increased progression of lung lesions. Efforts to find a clinical correlate of pathology findings, such as those from a high-resolution CT scan, would be beneficial and are needed in order to study the clinical utility of this association.
Our tissue pH results are the first lung tissue pH measurements among patients with pulmonary TB reported since 1953. Our results showing an acidic and favorable extracellular environment (median pH, 5.5) for the activation of pyrazinamide are in contrast to the findings of Weiser et al., in which they found tissue pHs ranging from 6.1 to 7.2 (15). For their measurements, they tested the supernatant from frozen and subsequently homogenized resected lung tissue, whereas we measured pH in the center of the resected lesions, specifically targeting the liquefied caseum, directly after surgery. Further study and validation of lung tissue pH measurements among TB patients are needed to obtain a better understanding of the extracellular conditions at the site of disease; such data are especially important to determine the activity of pH-dependent drugs, such as pyrazinamide, which is up to 20 times more active at a pH of 5.5 than at a pH of 6.8 (16). If chronic lung lesions due to TB maintain a low-pH environment for a prolonged period, as our findings suggest, this would provide a rationale for potentially continuing pyrazinamide for longer than 2 months.
In agreement with the findings of a study by Lanoix et al. with mice, we did not find pyrazinamide drug resistance mutations to explain the persistent growth of M. tuberculosis (11). In nonresponding C3HeB/FeJ mice, Lanoix et al. found that pyrazinamide-resistant M. tuberculosis isolates from lung caseous lesions never exceeded 1% of the total population, which is in line with the lack of pncA or rpsA drug resistance-associated mutations among 11 M. tuberculosis isolates from our two patients with positive tissue cultures (11). There are various possible explanations for the persistent culture positivity in our two patients, including the presence of highly drug-resistant (pre-XDR and XDR) M. tuberculosis isolates and the use of inadequate treatment regimens. It is also possible that the neutral tissue pH (7.2) found in one patient contributed to pyrazinamide inactivity and persistent organism growth in culture. While tissue pyrazinamide concentrations were not available for this patient, the serum free pyrazinamide concentration was high at 41.04 μg/ml, and even if assuming a low tissue penetration ratio of 0.54, a low tissue concentration is an unlikely explanation.
Our study results demonstrate that when the pyrazinamide dose is selected on the basis of the WHO-endorsed dose of 25 mg/kg, peak pyrazinamide concentrations with the recommended range of 20 to 60 μg/ml can reliably be achieved. However, there is a renewed debate on whether higher serum concentrations of pyrazinamide would be beneficial. Early studies using daily doses of pyrazinamide of between 30 and 50 mg/kg indicated that they had higher efficacy, but concerns over hepatotoxicity led to the use of a decreased dose (17). A recent analysis of data from three clinical trials evaluating high-dose rifampin found a steep exposure-response relationship between pyrazinamide Cmaxs (range, 15 to 55 μg/ml) and the time to sputum culture conversion, irrespective of the rifampin dose (18). If these results are confirmed and a higher pyrazinamide dose is shown to be well tolerated, the use of higher pyrazinamide doses is likely to be revisited. In the scenario of aiming for increased pyrazinamide exposure, our results along with those described in previously published reports suggest that one can predictably achieve a higher serum concentration with a higher weight-based dose (17, 19). Similar to the findings of a prior study, it is also important to note that we found a high degree of variability in pharmacokinetic parameters, including clearance and the volume of distribution, among our small sample of patients (20). Larger studies will be needed to study predictors of pyrazinamide pharmacokinetic parameters.
Our study is subject to certain limitations. These include the measurement of tissue pyrazinamide concentrations at only one time point and only one location within the resected lesion. We timed the administration of pyrazinamide on the day of surgery to correlate with the expected time to the maximum concentration in serum; however, it is not known if the time to the maximum concentration in tissue is similar or whether there is a delay which could have potentially led to an underestimation of tissue pyrazinamide concentrations. Additionally, our microdialysis approach allowed us to obtain free pyrazinamide concentrations at only one intralesional location, preventing us from determining the lesional distribution of pyrazinamide. We targeted the caseous center of resected lesions, as this has been considered the area with numerous extracellular bacilli and a limited immune response (21). It is also unclear how the delay from lung resection to the measurement of tissue pH (∼3 h) may have affected the pH results. The targeting of the relatively acellular caseous center of resected lesions is likely to have limited any effect of cellular death on lesion pH. To resolve this uncertainty, we have implemented the use of a micro-pH electrode and will compare tissue pH readings obtained intraoperatively using the electrode to measures taken 3 h later with both the electrode and pH test strips. In regard to lung tissue culture results, the use of solid versus liquid culture medium may have decreased the sensitivity of detection of M. tuberculosis growth, while the discordance between tissue acid-fast bacillus (AFB) smear and culture results may have been a result of M. tuberculosis organisms that were either nonviable or in a metabolically dormant nonculturable state (22). Further study would be required to address these culture-based questions and their implications.
In summary, our results provide encouraging data in regard to both the reliable and good tissue penetration of pyrazinamide into the diseased lung and the favorable acidic environment of most chronic tuberculous lesions, which promotes the bactericidal and sterilizing activity of pyrazinamide. These data offer confirmation of and a possible rationale for the importance of pyrazinamide in treatment regimens for both drug-susceptible and -resistant TB and its inclusion in most new drug combinations being tested.
MATERIALS AND METHODS
Study population.
Study participants were enrolled at the National Center for Tuberculosis and Lung Diseases (NCTLD) in Tbilisi, Georgia. Patients with culture-confirmed TB who were receiving pyrazinamide and scheduled to undergo adjunctive surgical resection were included. Treatment regimens were individualized on the basis of drug susceptibility testing (DST) results per WHO and local guidelines (23). All treatments were given through directly observed therapy. For pyrazinamide dosing, patients weighing ≤55 kg received 1,200 mg daily, while those weighing >55 kg received 1,600 mg daily. On the day of surgery, pyrazinamide was given orally with a few milliliters of water. The recommendation to perform adjunctive surgery was made by the NCTLD Drug Resistance Committee, as previously described and following recommendations from international guidelines (13, 23–25). All participants provided informed consent, and the study was approved by the NCTLD, Emory University, and University of Florida Institutional Review Boards.
Pharmacokinetics.
Patients fasted overnight prior to surgery and received pyrazinamide approximately 2 h before surgical resection. Serum samples were collected before and 1, 4, and 8 h after the patients received pyrazinamide and at the time of resection. Serum samples were kept in a −80°C freezer until they were shipped to the University of Florida Infectious Diseases Pharmacokinetics Laboratory (IDPL), Gainesville, FL, USA. Concentrations were measured using a validated liquid chromatography-tandem mass spectrometry (LC-MS-MS) assay on a Thermo Acella high-performance liquid chromatography system and a Thermo Ultra triple-quadrupole mass spectrometer with a Dell computer and the Thermo Xcaliburdata management system. The pyrazinamide concentration on the six-point standard curve ranged from 2 to 100 μg/ml, with linearity extending above and below this range. The recovery of pyrazinamide from human plasma was approximately 91%. The overall validation precision for pyrazinamide quality control samples was 2.92 to 15.12%. A modification of this assay was used to measure the pyrazinamide concentration in microdialysis samples. The samples used to prepare the standard curves for these analyses were diluted in saline.
Microdialysis (μD) was performed ex vivo on resected tissue specimens immediately after surgical removal, as previously described (13). Briefly, a semipermeable μD probe with a total length of 10 mm attached to a μD infusion pump (μ Dialysis AB, Stockholm, Sweden) was inserted into the center of the resected lesion using a slit cannula introducer. Four different concentrations of pyrazinamide (5, 10, 30, and 50 μg/ml) in Ringer's solution were each infused for approximately 35 min at a flow rate of 1 μl/min, and the recovered fluid dialysate was collected in microvials and then stored in a −80°C freezer until shipment to IDPL.
Laboratory.
All sputum and tissue acid-fast bacillus (AFB) smear microscopy and culture analyses were performed at the NCTLD National Reference Laboratory using standard methodologies. For each patient, a sputum sample and five tissue samples for culture from the resected lung lesion and surrounding tissue were collected preoperatively. Prior to culture, all tissue samples were first homogenized with a tissue grinder before inoculation on Lowenstein-Jensen (LJ)-based solid medium. For cultures with growth of M. tuberculosis, first- and second-line DST was performed as previously described (26, 27). DST for pyrazinamide was not performed.
DNA extraction was performed using a QIAamp DNA minikit (Qiagen Inc., Valencia, CA) for all available tissue and sputum specimens culture positive for M. tuberculosis. Extracted DNA was frozen at NCTLD until shipment to Emory University, where whole-genome sequencing was performed using an Illumina HiSeq 2000 instrument. The FastQ sequencing files for all M. tuberculosis isolates were uploaded to the Phylo-Resistance Search Engine (PhyResSE; http://phyresse.org, accession numbers 593375378609f78d466ecec7ceff6768 and 75ba5a7139084e9f061875f303b6d822), which is a web-based tool to delineate the M. tuberculosis antibiotic resistance and lineage from whole-genome sequencing data (28).
After microdialysis, a pH test strip was inserted into the center of the bisected lesion for pH measurement. Subsequently, one half of the lesion was formalin fixed and paraffin embedded. Four-micrometer sections were stained with hematoxylin and eosin and acid-fast (Fite) stains. Histopathology was used to assess the amount of inflammation (mononuclear cells, including multinucleated giant cells and polymorphonuclear neutrophils), necrosis, fibrosis, vascularization, and hemorrhage and the amount and location of acid-fast bacilli. The amount of necrosis was quantified as rare when necrosis was scattered and confined to one block, moderate when there were confluent areas in multiple blocks, and severe when the confluent areas of necrosis spanned several fields in a block and were present in multiple blocks. The amount of organisms was quantified as small when 1 to 5 organisms were observed in the submitted slides and large when there were abundant organisms.
Radiology.
Preoperative chest computed tomography (CT) scans, when available, were reviewed independently by two Emory University chest radiologists. The dominant abnormality from the resected lung was described by lesion type (cavity, mass, or consolidation), dimension, presence of calcification, and connection to the bronchus, and for cavitary lesions, the maximum wall thickness was measured. Any lesion that contained a gas-filled area was defined as a cavity, whereas a solid space-occupying lesion without a gas-filled area was defined as a mass lesion. Lesions characterized by replacement of the alveolar space with liquid were defined as a consolidation.
Data analysis.
Data analyses were performed using SAS software, and for noncompartmental pharmacokinetic analysis, Phoenix WinNonlin software was utilized. The following pharmacokinetic parameters were determined: maximal serum concentration (Cmax), the time at which Cmax occurred (Tmax), the area under the serum concentration-versus-time curve (AUC), volume of distribution divided by bioavailability (V/F), clearance over bioavailability (CL/F), half-life (t1/2), and elimination constant (kel). The fraction of the dose absorbed (F) was assumed to be 1 for data analysis. Serum free pyrazinamide concentrations were calculated by multiplying the measured serum pyrazinamide concentration by (100% to 15% [the midpoint of the range provided in the package insert for pyrazinamide]) (29). In comparing serum and tissue free drug concentrations, the serum concentration from the time of surgical resection was used. For one patient, a one-compartment model was used to calculate the serum pyrazinamide concentration at the time of surgical resection, as this sample was unavailable.
Accession number(s).
All sequencing data were deposited in the NCBI Sequence Read Archive (SRA) under accession number SRP102071.
Supplementary Material
ACKNOWLEDGMENTS
No author has a commercial or other association that might pose a conflict of interest.
This work was supported in part by the National Institutes of Health Fogarty International Center (D43TW007124), the National Institute of Allergy and Infectious Diseases (K23AI103044, R21AI122001), the Atlanta Clinical and Translational Science Institute (UL1TR000454), and the Emory University Global Health Institute.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00226-17.
REFERENCES
- 1.World Health Organization. 2016. Global tuberculosis report 2016. Report WHO/HTM/TB/2016.13. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.World Health Organization. 2015. Implementing the end TB strategy: the essentials. WHO/HTM/TB/2015.31 World Health Organization, Geneva, Switzerland. [Google Scholar]
- 3.Lienhardt C, Kraigsley AM, Sizemore CF. 2016. Driving the way to tuberculosis elimination: the essential role of fundamental research. Clin Infect Dis 63:370–375. doi: 10.1093/cid/ciw250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kempker RR, Kipiani M, Mirtskhulava V, Tukvadze N, Magee MJ, Blumberg HM. 2015. Acquired drug resistance in Mycobacterium tuberculosis and poor outcomes among patients with multidrug-resistant tuberculosis. Emerg Infect Dis 21:992–1001. doi: 10.3201/eid2106.141873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamilton CD, Stout JE, Goodman PC, Mosher A, Menzies R, Schluger NW, Khan A, Johnson JL, Vernon AN, Tuberculosis Trials Consortium. 2008. The value of end-of-treatment chest radiograph in predicting pulmonary tuberculosis relapse. Int J Tuberc Lung Dis 12:1059–1064. [PMC free article] [PubMed] [Google Scholar]
- 6.Kim HR, Hwang SS, Kim HJ, Lee SM, Yoo CG, Kim YW, Han SK, Shim YS, Yim JJ. 2007. Impact of extensive drug resistance on treatment outcomes in non-HIV-infected patients with multidrug-resistant tuberculosis. Clin Infect Dis 45:1290–1295. doi: 10.1086/522537. [DOI] [PubMed] [Google Scholar]
- 7.Azeredo FJ, Dalla Costa T, Derendorf H. 2014. Role of microdialysis in pharmacokinetics and pharmacodynamics: current status and future directions. Clin Pharmacokinet 53:205–212. doi: 10.1007/s40262-014-0131-8. [DOI] [PubMed] [Google Scholar]
- 8.Cornett DS, Reyzer ML, Chaurand P, Caprioli RM. 2007. MALDI imaging mass spectrometry: molecular snapshots of biochemical systems. Nat Methods 4:828–833. doi: 10.1038/nmeth1094. [DOI] [PubMed] [Google Scholar]
- 9.Kjellsson MC, Via LE, Goh A, Weiner D, Low KM, Kern S, Pillai G, Barry CE III, Dartois V. 2012. Pharmacokinetic evaluation of the penetration of antituberculosis agents in rabbit pulmonary lesions. Antimicrob Agents Chemother 56:446–457. doi: 10.1128/AAC.05208-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanoix JP, Lenaerts AJ, Nuermberger EL. 2015. Heterogeneous disease progression and treatment response in a C3HeB/FeJ mouse model of tuberculosis. Dis Model Mech 8:603–610. doi: 10.1242/dmm.019513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanoix JP, Ioerger T, Ormond A, Kaya F, Sacchettini J, Dartois V, Nuermberger E. 2016. Selective inactivity of pyrazinamide against tuberculosis in C3HeB/FeJ mice is best explained by neutral pH of caseum. Antimicrob Agents Chemother 60:735–743. doi: 10.1128/AAC.01370-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Shi W, Zhang W, Mitchison D. 2014. Mechanisms of pyrazinamide action and resistance. Microbiol Spectr 2:MGM2-0023-2013. doi: 10.1128/microbiolspec.MGM2-0023-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kempker RR, Barth AB, Vashakidze S, Nikolaishvili K, Sabulua I, Tukvadze N, Bablishvili N, Gogishvili S, Singh RS, Guarner J, Derendorf H, Peloquin CA, Blumberg HM. 2015. Cavitary penetration of levofloxacin among patients with multidrug-resistant tuberculosis. Antimicrob Agents Chemother 59:3149–3155. doi: 10.1128/AAC.00379-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prideaux B, Via LE, Zimmerman MD, Eum S, Sarathy J, O'Brien P, Chen C, Kaya F, Weiner DM, Chen PY, Song T, Lee M, Shim TS, Cho JS, Kim W, Cho SN, Olivier KN, Barry CE III, Dartois V. 2015. The association between sterilizing activity and drug distribution into tuberculosis lesions. Nat Med 21:1223–1227. doi: 10.1038/nm.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weiser OL, Howard OP, Dye WE. 1953. Assay of streptomycin in resected lung tissue, p 198–201. Trans 12th Conf Chemother Tuberc. [Google Scholar]
- 16.Zhang Y, Permar S, Sun Z. 2002. Conditions that may affect the results of susceptibility testing of Mycobacterium tuberculosis to pyrazinamide. J Med Microbiol 51:42–49. doi: 10.1099/0022-1317-51-1-42. [DOI] [PubMed] [Google Scholar]
- 17.Donald PR, Maritz JS, Diacon AH. 2012. Pyrazinamide pharmacokinetics and efficacy in adults and children. Tuberculosis (Edinb) 92:1–8. doi: 10.1016/j.tube.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Savic R, Peloquin CA, Boeree M, Weiner M, Heinrich N, Bliven-Sizemore E, Whitworth W, Morlock G, Posey J, MacKenzie W, Aarntouse R, Dooley K. 2016. The relationship between pyrazinamide pharmacokinetics (PK) and microbiologic outcomes in patients with pulmonary TB receiving standard- or high-dose rifampicin: PK/PD results from TBTC trials 27 and 28 and PanACEA MAMS, abstr O_12 Abstr 9th Int Workshop Clin Pharmacol Tuberc Drugs, Liverpool, United Kingdom. [Google Scholar]
- 19.Ellard GA. 1969. Absorption, metabolism and excretion of pyrazinamide in man. Tubercle 50:144–158. doi: 10.1016/0041-3879(69)90020-8. [DOI] [PubMed] [Google Scholar]
- 20.Zhu M, Starke JR, Burman WJ, Steiner P, Stambaugh JJ, Ashkin D, Bulpitt AE, Berning SE, Peloquin CA. 2002. Population pharmacokinetic modeling of pyrazinamide in children and adults with tuberculosis. Pharmacotherapy 22:686–695. doi: 10.1592/phco.22.9.686.34067. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan G, Post FA, Moreira AL, Wainwright H, Kreiswirth BN, Tanverdi M, Mathema B, Ramaswamy SV, Walther G, Steyn LM, Barry CE III, Bekker LG. 2003. Mycobacterium tuberculosis growth at the cavity surface: a microenvironment with failed immunity. Infect Immun 71:7099–7108. doi: 10.1128/IAI.71.12.7099-7108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chengalroyen MD, Beukes GM, Gordhan BG, Streicher EM, Churchyard G, Hafner R, Warren R, Otwombe K, Martinson N, Kana BD. 2016. Detection and quantification of differentially culturable tubercle bacteria in sputum from patients with tuberculosis. Am J Respir Crit Care Med 194:1532–1540. doi: 10.1164/rccm.201604-0769OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization. 2014. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. Report WHO/HTM/TB/2014.11. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 24.Vashakidze S, Gogishvili S, Nikolaishvili K, Dzidzikashvili N, Tukvadze N, Blumberg HM, Kempker RR. 2013. Favorable outcomes for multidrug and extensively drug resistant tuberculosis patients undergoing surgery. Ann Thorac Surg 95:1892–1898. doi: 10.1016/j.athoracsur.2013.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Partners in Health. 2003. Adjuvant therapies and strategies. In The PIH guide to the medical management of multidrug-resistant tuberculosis, p 29–32. Partners in Health, Boston, MA. [Google Scholar]
- 26.Parsons LM, Somoskovi A, Gutierrez C, Lee E, Paramasivan CN, Abimiku A, Spector S, Roscigno G, Nkengasong J. 2011. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clin Microbiol Rev 24:314–350. doi: 10.1128/CMR.00059-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tukvadze N, Kempker RR, Kalandadze I, Kurbatova E, Leonard MK, Apsindzelashvili R, Bablishvili N, Kipiani M, Blumberg HM. 2012. Use of a molecular diagnostic test in AFB smear positive tuberculosis suspects greatly reduces time to detection of multidrug resistant tuberculosis. PLoS One 7:e31563. doi: 10.1371/journal.pone.0031563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feuerriegel S, Schleusener V, Beckert P, Kohl TA, Miotto P, Cirillo DM, Cabibbe AM, Niemann S, Fellenberg K. 2015. PhyResSE: a web tool delineating Mycobacterium tuberculosis antibiotic resistance and lineage from whole-genome sequencing data. J Clin Microbiol 53:1908–1914. doi: 10.1128/JCM.00025-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woo J, Cheung W, Chan R, Chan HS, Cheng A, Chan K. 1996. In vitro protein binding characteristics of isoniazid, rifampicin, and pyrazinamide to whole plasma, albumin, and alpha-1-acid glycoprotein. Clin Biochem 29:175–177. doi: 10.1016/0009-9120(95)02024-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.