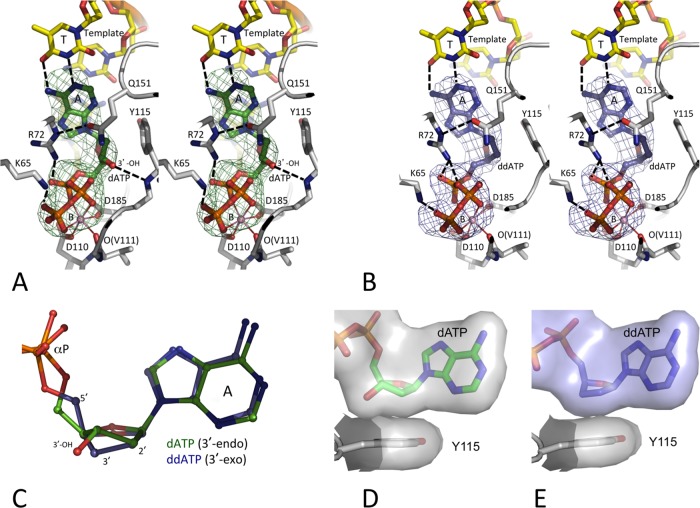
FIG 2.
Binding of dATP versus ddATP to the HIV-1 RT/DNA complex. The positioning of dATP (green) (A) and ddATP (blue) (B), the surrounding amino acid residues (gray carbon), and the DNA template/primer (yellow carbon) in the crystal structures were ascertained by difference Fourier (Fo − Fc) maps calculated prior to inclusion of the dATP (or ddATP) into the refinement and are displayed at 3σ (Fo and Fc are the amplitudes of observed and calculated structure factors, respectively). The chelation of the catalytic metal ion B is shown as thin solid lines, and hydrogen bonds are shown as dashed lines. Like in most published RT/DNA ternary complexes (10, 31, 51, 52), dATP (or ddATP) molecules in the current structures chelate one Mg2+ ion (metal B) with a superimposable coordination geometry involving one oxygen each from the three (α, β, and γ) phosphates. Ion B also chelates the main chain carbonyl oxygen of V111 and one carboxyl oxygen of the catalytic residues D110 and D185 to complete an octahedral coordination. This coordination environment is invariant in all available structures of RT/DNA/dNTP ternary complexes (7). The images in panels A and B are in stereo. (C) An active-site superposition of the two structures reveals 3′-endo and 3′-exo conformations of RT-bound dATP and ddATP, respectively. (D and E) The hydrophobic interactions of dATP (D) and ddATP (E) with Y115 shows that the lack of the 3′-OH in ddATP is partly compensated for by the 3′-exo conformation of the sugar ring.