FIG 3.
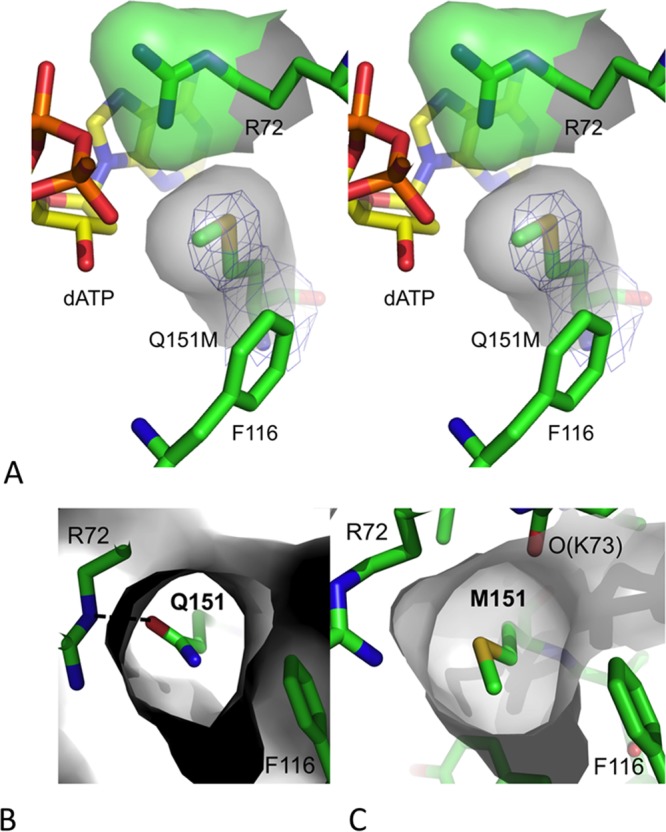
Conformation of M151 in the Q151M RT/DNA/dATP complex. (A) A stereo view showing the position and location of mutated residue Q151M in the structure of complex III defined by electron density (blue mesh). The Q151M mutation altered the side chain torsion of conserved residue R72. The van der Waals surfaces of R72 and M151 show the complementarity in the positioning of the two residues in the dNTP-binding site of the Q151M ternary structure. (B and C) van der Waals surfaces (in gray) surrounding Q151 (B) and M151 (C) in the structures of wtRT and Q151M complexes indicate that the flexible M151 side chain, unlike the Q151 side chain in wtRT, may alter the position of its C-ε atom by varying the torsion χ3 and perturbing the conformation of dNTP binding.
