ABSTRACT
Free-living amoebae of the genus Acanthamoeba are the causal agents of a sight-threatening ulceration of the cornea called Acanthamoeba keratitis, as well as the rare but usually fatal disease granulomatous amoebic encephalitis. Although there are many therapeutic options for the treatment of Acanthamoeba infections, they are generally lengthy and/or have limited efficacy. For the best clinical outcome, treatments should target both the trophozoite and the cyst stages, as cysts are known to confer resistance to treatment. In this study, we document the activities of caffeine and maslinic acid against both the trophozoite and the cyst stages of three clinical strains of Acanthamoeba. These drugs were chosen because they are reported to inhibit glycogen phosphorylase, which is required for encystation. Maslinic acid is also reported to be an inhibitor of extracellular proteases, which may be relevant since the protease activities of Acanthamoeba species are correlated with their pathogenicity. We also provide evidence for the first time that both drugs exert their anti-amoebal effects through programmed cell death.
KEYWORDS: Acanthamoeba; caffeine, maslinic acid, programmed cell death; PCD
INTRODUCTION
Members of the genus Acanthamoeba are ubiquitous protists and include the causative agents of several infections in humans such as a sight-threating ulceration of the cornea known as Acanthamoeba keratitis (AK), the usually fatal granulomatous amoebic encephalitis (GAE), and also a range of disseminated infections, usually, but not exclusively, limited to the skin (1–4).
The life cycle of Acanthamoeba alternates between two stages: the trophozoite, which is the active growing stage, and the cyst which is a dormant stage resorted to when conditions become incompatible with growth. The cyst has a highly resistant double wall. The outer wall is fibrous and composed mainly of protein while the inner wall contains more than 30% cellulose (2, 5, 6, 7). In AK, the cysts are responsible for recurrent amoebic infections, as it is able to survive many of the current treatments (8, 9) and differentiate back to amoebae on the cessation of treatment. Diamidines (proamidine and hexamidine) and biguanides (chlorhexidine and polyhexamethylene biguanide [PHMB]), have been found to be effective against Acanthamoeba trophozoites and cysts in vitro (2, 3, 4, 10). However, it has been reported that about 5% of patients with AK are troubled with inflammation due to the persistence of Acanthamoeba infection caused by cysts surviving in the cornea, even after prolonged treatment with these agents (11). There is an urgent need for more effective treatments and so there is also a need to identify and validate new therapeutic targets in Acanthamoeba, mostly focusing on key proteins related to cellular viability and the pathogenic mechanisms. In this report we explore two such targets, secreted proteases and glycogen phosphorylase.
Acanthamoeba secretes three types of proteolytic enzymes: serine proteases, cysteine proteases, and metalloproteases, and these cause at least part of the organism's pathogenicity (12). RNA interference (RNAi) silencing experiments confirm this (13, 14), and it has been demonstrated that serine proteases play a role in the important processes of encystment and excystation (13, 15, 16).
Glycogen phosphorylase is active during encystation in breaking down glycogen to release glucose-1-phosphate, a precursor of the cellulose required to construct the inner cyst wall. We have shown that the inhibition of this enzyme by RNAi blocks the formation of cysts (14). However, since RNAi is not yet widely approved, a drug which performs the same function is preferable in the treatment of AK.
Caffeine and maslinic acid are reported to be glycogen phosphorylase inhibitors (17, 18). Maslinic acid is a natural triterpene isolated from the olive tree (Olea europaea) with multiple biological properties, such as antimicrobial and antiparasitic activity (19, 20, 21, 22). Maslinic acid has been reported to be a potent inhibitor of glycogen phosphorylase and extracellular proteases of parasites such as Toxoplasma gondii and serine proteases from Cryptosporidium (19, 23, 24, 25, 26).
RESULTS
Caffeine and maslinic acid were both amoebicidal and cysticidal.
Caffeine and maslinic acid were both active against the trophozoite stage of different strains of Acanthamoeba. Caffeine had higher activity than maslinic acid (except against strain CLC-16) (Table 1). Although both products seemed to have a lower activity than chlorhexidine, their activity was still higher that of amphotericin B (Table 1).
TABLE 1.
The alamarBlue cell viability assay was used to determine IC50 and IC90 values after 96 h for caffeine and maslinic acid tested against the four strains of Acanthamoeba
| Treatment | ICs (μM) for Acanthamoeba strains |
|||||||
|---|---|---|---|---|---|---|---|---|
| AcNeffa |
CLC-16 |
CLC-41.r |
CLC-51.l |
|||||
| IC50 | IC90 | IC50 | IC90 | IC50 | IC90 | IC50 | IC90 | |
| Caffeine | 8.86 ± 2.11 | 22.71 ± 0.93 | 42.94 ± 4.22 | 72.47 ± 5.07 | 24.30 ± 2.01 | 52.25 ± 6.22 | 17.35 ± 0.72 | 45.65 ± 2.05 |
| Maslinic acid | 47.60 ± 0.53 | 85.68 ± 4.38 | 22.04 ± 2.28 | 37.15 ± 3.99 | 78.68 ± 6.05 | 103.78 ± 6.00 | 52.83 ± 2.76 | 83.75 ± 4.19 |
| Amphotericin B | 39.65 ± 0.56 | 100.01 ± 9.52 | 27.41 ± 1.28 | 71.89 ± 3.40 | 59.95 ± 1.05 | 145.44 ± 3.84 | 67.30 ± 5.68 | 170.11 ± 13.72 |
| Chlorhexidine | 5.23 ± 0.55 | 16.14 ± 0.90 | 11.89 ± 3.80 | 34.46 ± 2.43 | 2.45 ± 0.20 | 28.46 ± 4.33 | 10.47 ± 1.48 | 28.21 ± 1.80 |
AcNeff, Acathamoeba Neff type strain (ATCC 30010, genotype T4).
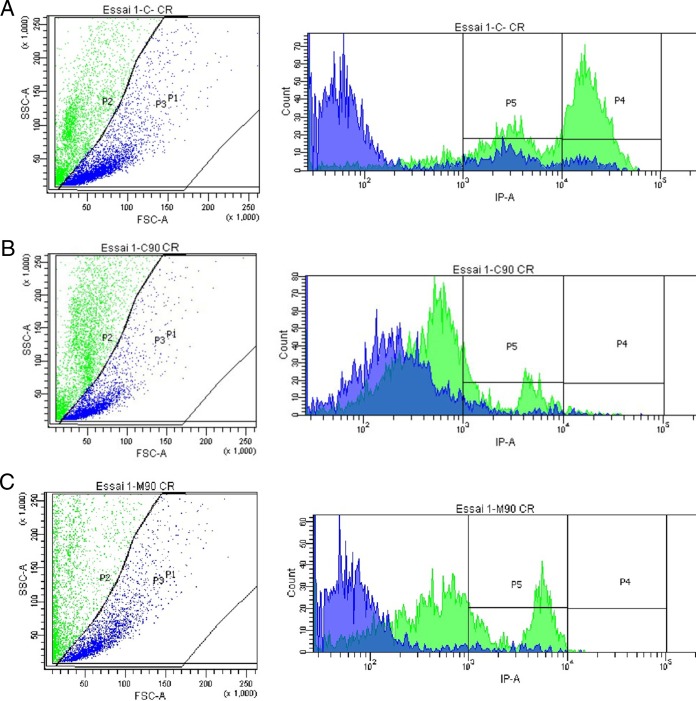
When cells were grown in encystation medium and stained with Congo red in order to analyze by flow cytometry, control cells were clearly divided into 3 main populations: P3 trophozoites, P4 cysts stained with Congo red, and P5 precysts stained with Congo red (Fig. 1A). However, after treatments using caffeine and maslinic acid, trophozoites were unable to either encyst or form mature cysts (Fig. 1B and C).
FIG 1.
The Acanthamoeba Neff strain separated into subpopulations after 72 h, as follows: P1, total cell population, analyzed by the flow cytometer; P2, cysts; P3, trophozoites, analyzed according to size and complexity of cells; P4, cysts; P5, precysts, analyzed by fluorescence emitted by the Congo red staining. (A) Control. (B) Cells after treatment with caffeine IC90. (C) Cells after treatment with maslinic acid IC90.
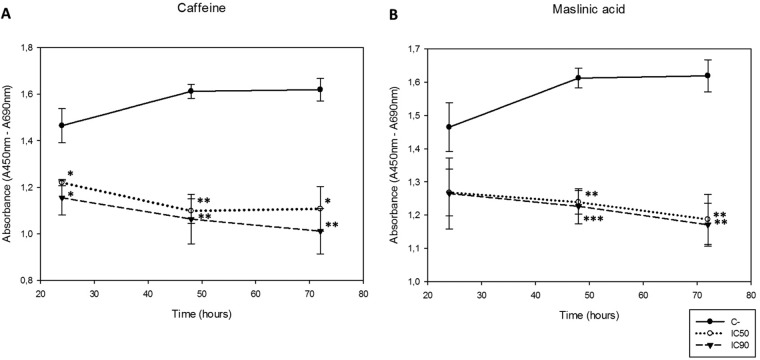
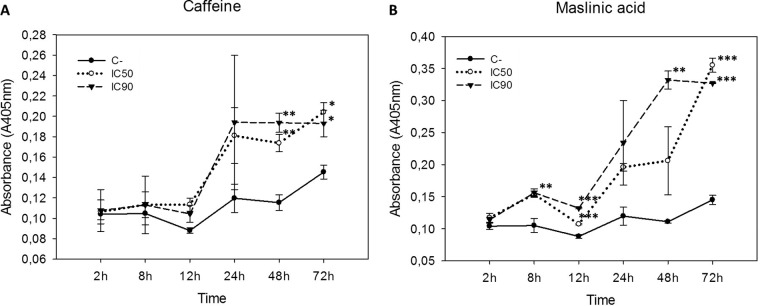
The effects of caffeine and maslinic acid on cell proliferation from 24 to 72 h were checked. It was noted that both active principles decreased cell proliferation in a dose-dependent manner (Fig. 2). Furthermore, significant differences between the control and the 50% and 90% inhibitory concentrations (IC50 and IC90, respectively) were observed, except at 24 h when maslinic acid was used (Fig. 2B). No significant differences between both inhibitory concentrations were observed, which may serve to establish the IC50 as sufficient to eliminate the cell population.
FIG 2.
Observed cell proliferation after incubation of the Acanthamoeba Neff strain with caffeine and maslinic acid using the previously obtained IC50 and IC90 compared to the control. Results were analyzed at 24, 48, and 72 h. (A) Effects on cell proliferation when cells were treated with caffeine. (B) Effects on cell proliferation when cells were treated with maslinic acid. Statistical differences between both concentrations and the control were observed (***, P < 0.001; **, P < 0.01; *, P < 0.05).
Caffeine and maslinic acid showed low cytotoxicity to vertebrate cells.
The results showed that caffeine (both IC50 and IC90, C50 and C90, respectively, for Acanthamoeba) and maslinic acid IC50 (M50) were not cytotoxic toward HeLa or J774.A1 vertebrate cells. Caffeine and maslinic acid showed significantly low cytotoxicity compared to the reference drugs chlorhexidine and amphotericin B (Fig. 3).
FIG 3.
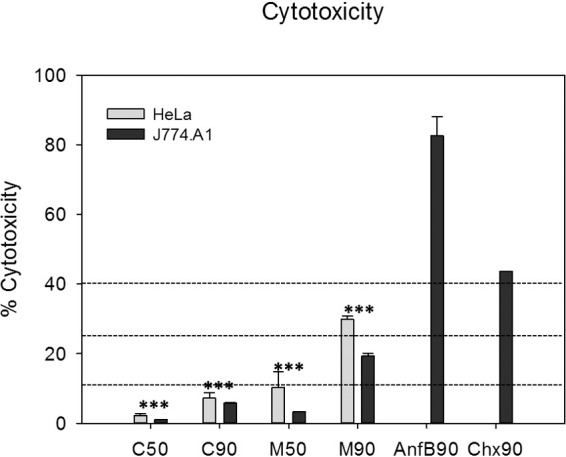
Cytotoxicity levels of the tested caffeine and maslinic acid against Acanthamoeba (IC50 and IC90) were evaluated against two cell lines: HeLa and murine macrophages. Values lower than 10% correspond to a null cytotoxicity, so the results showed that caffeine IC50 (C50), caffeine IC90 (C90), and maslinic acid IC50 (M50) were not cytotoxic. Values between 10% to 25% correspond to a low cytotoxicity, which was the case for maslinic acid IC90 (M90). Statistical differences between cytotoxicity of the assayed drugs and that produced by the reference drugs chlorhexidine IC90 (Chx90) and amphotericin B (Anf90) were observed in both cell lines (***, P < 0.001).
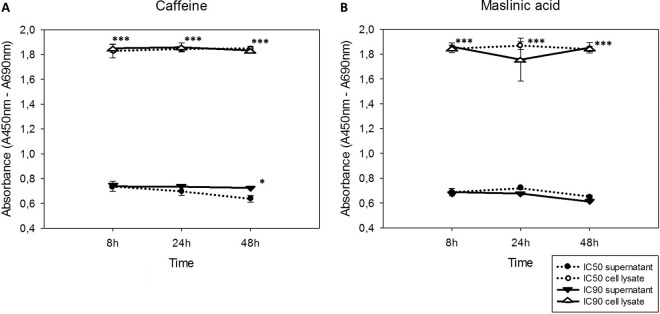
Caffeine and maslinic acid induced larger amounts of DNA in the cell lysate than in the supernatant.
When Acanthamoeba Neff was treated with the IC50 and IC90 of caffeine and maslinic acid, a larger amount of DNA was observed in the cell lysate compared to the detected levels in the supernatant (Fig. 4). Therefore, a larger amount of intracellular DNA was detected in all cases, with significant differences between the detected DNA in the lysate and the supernatant. In the case of caffeine (Fig. 4A), significant differences between the concentrations were also observed at 48 h after the treatment.
FIG 4.
Amount of DNA detected over time in the culture supernatant and cell lysate (absorbance versus time). (A) Caffeine. (B) Maslinic acid. Statistical differences (***, P < 0.001; *, P < 0.05) are shown for result comparison between supernatant (filled symbols) and cell lysate (empty symbols) values.
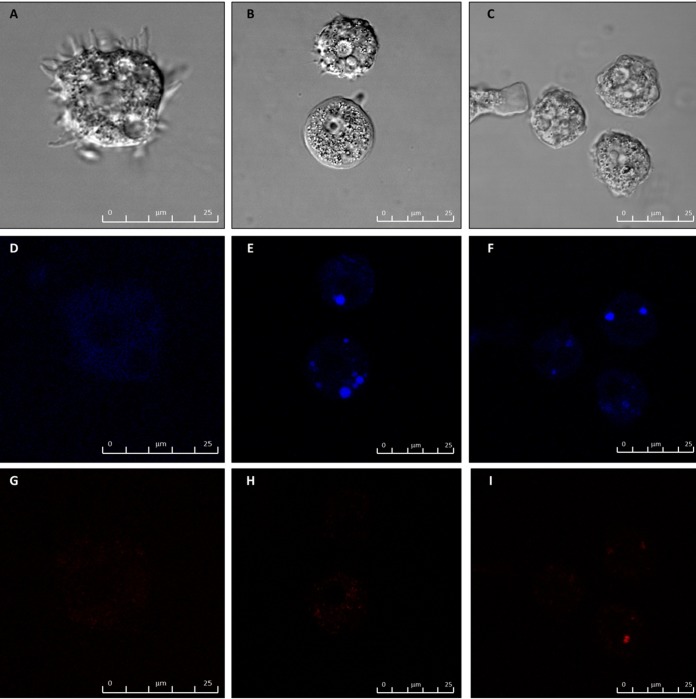
Caffeine and maslinic acid induced PCD, which was observed with double stain assay.
When double staining was performed, caffeine and maslinic acid caused nuclei staining with Hoechst stain, demonstrating the presence of condensed chromatin (Fig. 5). Moreover, the differences between the three cells population were clear and thus live cells were detected under fluorescence microscopy, as they showed faint blue nuclei against a dark cytoplasmic background stain (Fig. 5D), whereas cells displaying programmed cell death (PCD) presented bright blue nuclei due to karyopyknosis and chromatin condensation (Fig. 5E and F). Dead cells were not able to exclude propidium iodide, a DNA binding dye, and so the remnants of the nuclei in these dead cells stained red (Fig. 5G to I). These images show that both caffeine and maslinic acid caused PCD after 24 h.
FIG 5.
Hoechst staining is different in control cells, where uniformly faint blue nuclei are observed, and in treated cells (at 24 h), where the nuclei are bright blue. (A to C) Phase contrast where A is the control, B caffeine (IC50), and C maslinic acid (IC50). (D to F) Hoechst channel where D is the control, E caffeine, and F maslinic acid. (G to I) Propidium iodine channel where G is the control, H caffeine, and I maslinic acid. Bar, 25 μm.
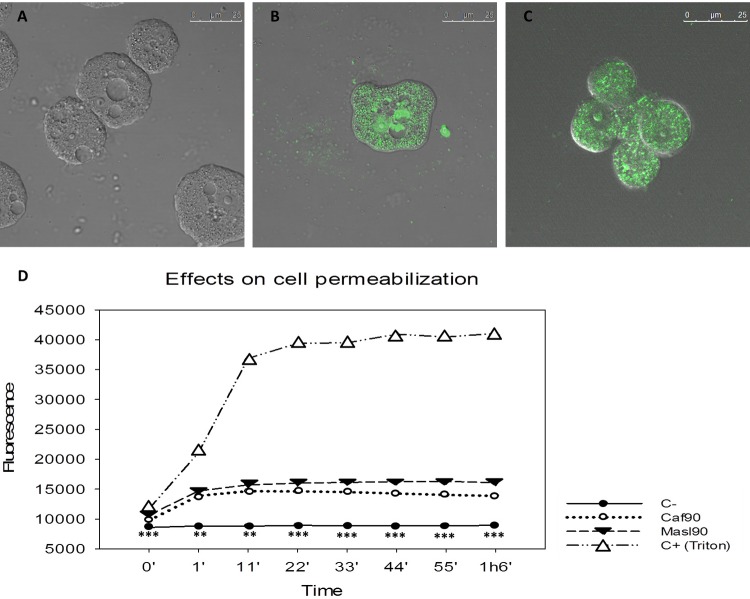
Caffeine and maslinic acid caused plasma membrane permeability.
Caffeine and maslinic acid induced cellular membrane damage in treated amoebae after 1 h of treatment. None of the tested products induced the same level of fluorescence observed in the positive control (Fig. 6D). Nevertheless, cellular membrane disruption was checked and confirmed using fluorescence microscopy in treated cells (Fig. 6A to C).
FIG 6.
Permeabilization of the cellular membrane. Fluorescence from the SYTOX Green nucleic acid stain can be observed when cells were treated with the different treatments after 2 h. (A) Control. (B) Caffeine. (C) Maslinic acid. (D) Differences between the total permeabilization of control (with addition of triton) and drug-treated cells were apparent when fluorescence of the cells was measured. Statistical differences (***, P < 0.001; **, P < 0.01) are shown for results obtained between negative control and the different treatments. Bar, 25 μm.
Amoebae treated with caffeine and maslinic acid showed signs of early PCD.
Acanthamoeba Neff treated with the assayed active principles showed externalization of phosphatidylserine (PS). The number of cells suffering PCD or death were counted and showed a clear difference between the control and the treated cells (Fig. 7). Moreover, the statistical analyses showed significant differences in the percentage of detected PCD cells after treatment with all the assayed drugs with respect to the control. Therefore, early stages of apoptosis in the treated Acanthamoeba cells were demonstrated.
FIG 7.
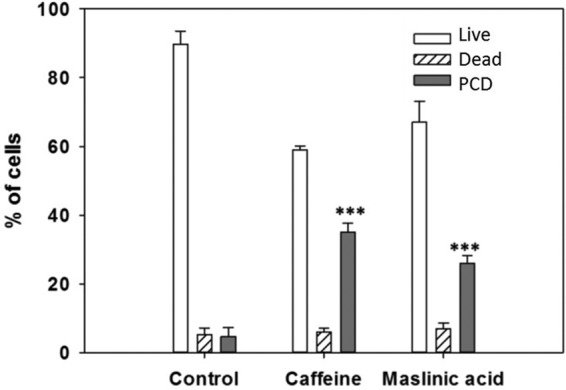
Histogram comparing cells and treatments. Results are represented in percentages and statistical differences (***, P < 0.001) are shown comparing apoptotic cells after treatments with the control.
Acanthamoeba caspase-3-like activity was detected after treatment with caffeine and maslinic acid.
A significant caspase-3-like activity was detected in amoebae treated with caffeine or maslinic acid using a chromogenic probe attached to a substrate peptide. Caspase-3 like activity developed in the presence of both drugs, especially after 24 h (Fig. 8).
FIG 8.
Caspase-like activity absorbance versus time (2 to 72 h). Statistical differences (***, P < 0.001; **, P < 0.01; ***, P < 0.05) are shown comparing control with the different treatment concentrations. (A) Caffeine. (B) Maslinic acid.
DISCUSSION
Caffeine and maslinic acid are glycogen phosphorylase inhibitors (17, 18) and maslinic acid inhibits the secreted proteases of a number of different parasites (19, 23, 24, 25, 26). The activity of these drugs had been successfully tested against different protozoa (19, 20, 23, 24, 25, 26) and maslinic acid has been found to be active against Acanthamoeba (22). In the present study, anti-Acanthamoeba activity of these drugs has been described using a range of Acanthamoeba strains. The fact that both drugs have anti-cyst activity was established by viability and proliferative assay and analyzed by flow cytometry in this study. The finding that both drugs blocked the development of cysts is compatible with their inhibitory effects on both proteases and the glycogen phosphorylase activity required for glucose release from glycogen to form the cellulose wall (6), but this does not explain caffeine's toxic effect on trophozoites. However, it is known that caffeine and maslinic acid induce apoptosis in various vertebrate cell types. For example, caffeine induce PCD in neuroblastoma cells and pancreatic and lung adenocarcinomas (27, 28, 29, 30) and maslinic acid induces apoptosis in metastatic cell lines and in colon cancer cells (31, 32). We could find no study in which either drug was reported to induce PCD in any protist.
PCD and PCD-like processes have been reported in a wide variety of protists (33), including Acanthamoeba (33, 34, 35, 36, 37). The present study has shown that caffeine and maslinic acid activate PCD and associated phenomena such as externalization of phosphatidylserine, chromatin condensation, and DNA fragmentation. We also found evidence for the involvement of a caspase-3-like enzyme since the well-known DEVD-p-nitroalanine caspase-3 substrate was cleaved in Acanthamoeba. Caspase-3 is an effector caspase that is responsible for DNA fragmentation, chromatin condensation, and membrane disruption. Other members of the family of caspases are the metacaspases and paracaspases. Paracaspases have been found in plants, fungi, and protozoa. Their function is not limited to cell death, but includes roles in sporulation and embryogenesis (38, 39, 40). In Acanthamoeba, a type-1 metacaspase has been identified. Its function is related to encystment (41), and activity relating to the osmoregulation processes has also been inferred (42). Our group has previously used the same method and found caspase-3-like activity stimulated by statin drugs in Acanthamoeba (34), and others have reported that violacein induces caspase-3 activity in Acanthamoeba (43), but we can find no obvious caspase-3 homologs in the various Acanthamoeba genome databases. It is possible that the Acanthamoeba enzyme that recognizes and cleaves this motif belongs to a different protease family.
Treatment that induces necrosis in parasites produces an inflammatory response in the host (44), so it is important to avoid the use of necrotic drugs in order to reduce inflammation in delicate tissues such as the eye in the case of AK, or the brain in GAE. Maslinic acid is well tolerated in mice (45) and rats (46) and we know that humans tolerate caffeine well, but it remains to be seen if either drug can be safely and comfortably introduced to the eye surface (or the brain) at the required concentration to be an effective treatment. The facts that maslinic acid inhibits encystment, is toxic to cysts and trophozoites, and the killing mechanism acts through PCD make maslinic acid an especially promising candidate for AK treatment.
MATERIALS AND METHODS
Acanthamoeba strains.
Three clinical isolates (CLC-16, genotype T3; CLC-41.r, genotype T4; and CLC-51, genotype T1) obtained in a previous study in our laboratory (47) and the type strain Acanthamoeba Neff (ATCC 30010, genotype T4) were used in this study. These strains were grown axenically in peptone yeast glucose (PYG) medium (0.75% [wt/vol] proteose peptone, 0.75% [wt/vol] yeast extract, and 1.5% [wt/vol] glucose) containing 40 μg/ml gentamicin (Biochrom AG, Cultek, Granollers, Barcelona, Spain) at room temperature.
Chemicals.
Two drugs were selected for the different experiments: caffeine (Sigma-Aldrich Chemistry Ltd.; Madrid, Spain) and maslinic acid (kindly provided by Instituto de Biotecnología, Department of Parasitology, University of Granada, Spain, and synthetized as previously described) (45). Results obtained with caffeine and maslinic acid were compared to results with chlorhexidine (chlorhexidine digluconate; Alfa Aesar) and amphotericin B (Sigma-Aldrich Chemistry Ltd.; Madrid, Spain), used as positive controls.
Activity assays.
The anti-trophozoite activities of the assayed drugs were determined by the alamarBlue assay as previously described (47, 48, 49).
Cysticidal activity.
The effects of drugs against cysts were evaluated by incubating Acanthamoeba Neff (105 cells/ml) with the previously calculated IC90 for each drug in Neff's encystment medium (which induces encystation) (NEM; 0.1 M KCl, 8 mM MgSO4·7H2O, 0.4 mM CaCl2·2H2O, 1 mM NaHCO3, 20 mM ammediol [2-amino-2-methyl-1,3-propanediol]; Sigma-Aldrich Chemistry Ltd., Madrid, Spain) pH 8.8, at 25°C. After 24, 48, and 72 h, samples were collected in flow cytometry tubes, where they were stained with the vital stain Congo red (Fisher Scientific) at 10 μg/ml for 30 min. This stain has a high affinity for cellulose, making it useful to stain mature cysts.
Samples were analyzed by flow cytometry using a BD FACSCanto II (Becton & Dickinson) driven by the manufacturer's software, BD FACS Diva. The different populations of cells were separated accordingly to size and complexity, as follows: P1 total population; P2 cysts; P3 trophozoites; P4 cysts stained with Congo red; P5 precysts stained with Congo red.
Cell proliferation.
In order to study the effect of the drugs on Acanthamoeba Neff cell proliferation, a Cell Proliferation ELISA, BrdU (colorimetric) kit (Roche) was used following the manufacture's recommendations and as previously described (49).
Mammalian cytotoxicity test.
The cytotoxicity produced by active compounds was evaluated against two mammalian cell lines, murine macrophages (ATCC TIB-67) and HeLa cells (ATCC CCL-2). A Cytotoxicity Detection kit (LDH) (Roche Applied Science) was used following the manufacture's recommendations. Results were classified based on previously established parameters. Drugs that have percentages of cytotoxicity between 0% to 10% were defined as being noncytotoxic, values between 10% to 25% as having low cytotoxicity, 25% to 40% as having moderate cytotoxicity, and values >40% having high cytotoxicity (14, 49).
Cellular DNA fragmentation.
A cellular DNA fragmentation kit (Roche) was used. This kit is an enzyme-linked immunosorbent assay (ELISA) for the detection of bromodeoxyuridine (BrdU)-labeled DNA fragments in culture supernatants and cellular lysates. The procedure for characterization of cell death consists of two parts: 1. Analysis of the supernatant, which contains DNA fragments at early stages of necrosis and late stages of apoptosis. 2. The remaining cells are lysed in order to release apoptotic DNA fragments located in the cytoplasm. The experiment was carried out following the manufacturer recommendations and as previously described (34).
Double stain assay for apoptosis determination.
A double stain apoptosis detection kit (Hoechst 33342/PI) (Genscript, Piscataway, NJ, USA) and an inverted confocal microscope (Leica DMI 4000B) were used. The experiment was carried out following the manufacturer recommendations and as previously described (34). 105 cells/well were incubated in a 24-well plate for 24 h with the previously calculated IC50 (Table 1). The double staining pattern allows the identification of three groups in a cellular population. Live cells show only a low level of blue Hoechst 33342 fluorescence, apoptotic cells show a higher level of blue fluorescence, and dead cells show low-blue and high-red propidium iodide (PI) fluorescence, as this dye only penetrates dead cells.
Plasma membrane permeability.
SYTOX Green nucleic acid stain (Invitrogen, Life Technologies SA, Madrid, Spain) is a high-affinity nucleic acid stain (absorption and emission maxima at 504 and 523 nm, respectively) that fluorescently stains permeable cells. The experiment was carried out following the manufacturer recommendations and as previously described (34). A positive control using 2.5% of Triton X-100 (Sigma) to fully permeabilize cells was included in the study.
Caspase-like activity detection.
A Caspase-3 Colorimetric Assay kit (Genscript, Piscataway, NJ, USA) was used following manufacturer recommendations and as in a previously described study (34). The assay is based on the chromophore p-nitroalanine, which is coupled to a peptide containing the caspase-3 substrate DEVD. On completion, the optical density (at 405 nm) of the experiment is compared to controls to determine caspase-3 activity.
Statistical analysis.
The obtained results were compared by one-way ANOVA, multiple post hoc analyses, and Tukey's test using the Sigma Plot 12.0 software (Systat Software).
ACKNOWLEDGMENTS
This work was supported by grants from RICET (project no. RD12/0018/0012 of the program of Redes Temáticas de Investigación Cooperativa, FIS), Spanish Ministry of Health, Madrid, Spain; project PI13/00490, “Protozoosis Emergentes por Amebas de Vida Libre: Aislamiento, Caracterización, Nuevas Aproximaciones Terapéuticas y Traslación Clínica de los Resultados” from the Instituto de Salud Carlos III; and projects AGUA3 “Amebas de Vida Libre como Marcadores de Calidad del Agua” and BIO24 “Principios Activos Inductores de Apoptosis en la Quimioterapia de Tripanosomosis y Leishmaniosis” from the CajaCanarias Fundación. A.L.-A. and I.S. were supported by the Agustín de Betancourt Programme.
REFERENCES
- 1.Marciano-Cabral F, Cabral G. 2003. Acanthamoeba spp. as agents of disease in humans. Clin Microbiol Rev 16:273–307. doi: 10.1128/CMR.16.2.273-307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuster FL, Visvesvara GS. 2004. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int J Parasitol 34:1001–1027. doi: 10.1016/j.ijpara.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqui R, Khan NA. 2012. Biology and pathogenesis of Acanthamoeba. Parasit Vectors 5:6. doi: 10.1186/1756-3305-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorenzo-Morales J, Martín-Navarro CM, López-Arencibia A, Arnalich-Montiel F, Piñero JE, Valladares B. 2013. Acanthamoeba keratitis: an emerging disease gathering importance worldwide? Trends Parasitol 29:181–187. doi: 10.1016/j.pt.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Dudley R, Alsam S, Khan NA. 2007. Cellulose biosynthesis pathway is a potential target in the improved treatment of Acanthamoeba keratitis. Appl Microbiol Biotechnol 75:133–140. doi: 10.1007/s00253-006-0793-8. [DOI] [PubMed] [Google Scholar]
- 6.Lorenzo-Morales J, Kliescikova J, Martínez-Carretero E, De Pablos LM, Profotova B, Nohynkova E, Osuna A, Valladares B. 2008. Glycogen phosphorylase in Acanthamoeba spp.: determining the role of the enzyme during the encystment process using RNA interference. Eukaryot Cell 7:509–517. doi: 10.1128/EC.00316-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemgruber L, Lupetti P, De Souza W, Vommaro RC, da Rocha-Azevedo B. 2010. The fine structure of the Acanthamoeba polyphaga cyst wall. FEMS Microbiology Lett 305:170–176. doi: 10.1111/j.1574-6968.2010.01925.x. [DOI] [PubMed] [Google Scholar]
- 8.Aksozek A, McClellan K, Howard K, Niederkorn JY, Alizadeh H. 2002. Resistance of Acanthamoeba castellanii cysts to physical, chemical, and radiological conditions. J Parasitol 88:621–623. doi: 10.1645/0022-3395(2002)088[0621:ROACCT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 9.Turner NA, Russell AD, Furr JR, Lloyd D. 2004. Resistance, biguanide sorption and biguanide-induced pentose leakage during encystment of Acanthamoeba castellanii. J Appl Microbiol 96:1287–1295. doi: 10.1111/j.1365-2672.2004.02260.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee JE, Oum BS, Choi HY, Yu HS, Lee JS. 2007. Cysticidal effect on Acanthamoeba and toxicity on human keratocytes by polyhexamethylene biguanide and chlorhexidine. Cornea 26:736–741. doi: 10.1097/ICO.0b013e31805b7e8e. [DOI] [PubMed] [Google Scholar]
- 11.Pérez-Santonja JJ, Kilvington S, Hughes R, Tufail A, Matheson M, Dart JK. 2003. Persistently culture positive acanthamoeba keratitis: in vivo resistance and in vitro sensitivity. Ophthalmology 110:1593–1600. doi: 10.1016/S0161-6420(03)00481-0. [DOI] [PubMed] [Google Scholar]
- 12.Khan NA, Jarroll EL, Panjwani N, Cao Z, Paget TA. 2000. Proteases as markers for differentiation of pathogenic and non-pathogenic species of Acanthamoeba. J Clin Microbiol 38:2858–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorenzo-Morales J, Ortega-Rivas A, Foronda P, Abreu-Acosta N, Ballart D, Martinez E, Valladares B. 2005. RNA interference (RNAi) for the silencing of extracellular serine proteases genes in Acanthamoeba: molecular analysis and effect on pathogenicity. Mol Biochem Parasitol 144:10–15. doi: 10.1016/j.molbiopara.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Lorenzo-Morales J, Martín-Navarro CM, López-Arencibia A, Santana-Morales MA, Afonso-Lehmann RN, Maciver SK, Valladares B, Martínez-Carretero E. 2010. Therapeutic potential of a combination of two gene-specific small interfering RNAs against clinical strains of Acanthamoeba. Antimicrob Agents Chemother 54:5151–5155. doi: 10.1128/AAC.00329-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dudley R, Alsam S, Khan NA. 2008. The role of proteases in the differentiation of Acanthamoeba castellanii. FEMS Microbiol Lett 286:9–15. doi: 10.1111/j.1574-6968.2008.01249.x. [DOI] [PubMed] [Google Scholar]
- 16.Leitsch D, Kohsler M, Marchetti-Deschmann M, Deutsch A, Allmaier G, Duchene M, Walochnik J. 2010. Major role for cysteine proteases during the early phase of Acanthamoeba castellanii encystment. Eukaryot Cell 9:611–618. doi: 10.1128/EC.00300-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsitsanou KE, Skamnaki VT, Oikonomakos NG. 2000. Structural basis of the synergistic inhibition of glycogen phosphorylase a by caffeine and a potential antidiabetic drug. Arch Biochem Biophys 384:245–254. doi: 10.1006/abbi.2000.2121. [DOI] [PubMed] [Google Scholar]
- 18.Freeman S, Bartlett JB, Convey G, Hardern I, Teague JL, Loxham SJG, Allen JM, Poucher SM, Charles AD. 2006. Sensitivity of glycogen phosphorylase isoforms to indole site inhibitors is markedly dependent on the activation state of the enzyme. Br J Pharmacol 149:775–785. doi: 10.1038/sj.bjp.0706925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Pablos LM, Gonzalez G, Rodrigues R, Garcia Granados A, Parra A, Osuna A. 2010. Action of a pentacyclic triterpenoid, maslinic acid, against Toxoplasma gondii. J Nat Prod 73:831–834. doi: 10.1021/np900749b. [DOI] [PubMed] [Google Scholar]
- 20.Moneriz C, Mestres J, Bautista JM, Diez A, Puyet A. 2011. Multi-targeted activity of maslinic acid as an antimalarial natural compound. FEBS J 278:2951–2961. doi: 10.1111/j.1742-4658.2011.08220.x. [DOI] [PubMed] [Google Scholar]
- 21.Sifaoui I, López-Arencibia A, Martín-Navarro CM, Ticona JC, Reyes-Batlle M, Mejri M, Jiménez AI, Lopez-Bazzocchi I, Valladares B, Lorenzo-Morales J, Abderabba M, Piñero JE. 2014. In vitro effects of triterpenic acids from olive leaf extracts on the mitochondrial membrane potential of promastigote stage of Leishmania spp. Phytomedicine 21:1689–1694. doi: 10.1016/j.phymed.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Sifaoui I, López-Arencibia A, Ticona JC, Martín-Navarro CM, Reyes-Batlle M, Mejri M, Lorenzo-Morales J, Jiménez AI, Valladares B, Lopez-Bazzocchi I, Abderabba M, Piñero JE. 2014. Bioassay guided isolation and identification of anti-Acanthamoeba compounds from Tunisian olive leaf extracts. Exp Parasitol 145(Suppl):S111–114. doi: 10.1016/j.exppara.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 23.García-Granados A, Martínez A, Parra A, Rivas F, Osuna A, Mascaró C, Rodríguez N, Kalifa L. 1997. Utilización de ácido maslínico como inhibidor de serín-proteasas para el tratamiento de enfermedades causadas por parásitos del género Cryptosporidium. Spanish patent P9701029.
- 24.Wen X, Sun H, Liu J, Wu G, Zhang L, Wu X, Ni P. 2005. Pentacyclic triterpenes. Part 1: the first examples of naturally occurring pentacyclic triterpenes as a new class of inhibitors of glycogen phosphorylases. Bioorg Med Chem Lett 15:4944–4948. doi: 10.1016/j.bmcl.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 25.Wen X, Zhang P, Liu J, Zhang L, Wu X, Ni P, Sun H. 2006. Pentacyclic triterpenes. Part 2: synthesis and biological evaluation of maslinic acid derivatives as glycogen phosphorylase inhibitors. Bioorg Med Chem Lett 16:722–726. doi: 10.1016/j.bmcl.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Chen J, Liu J, Zhang L, Wu G, Hua W, Wu X, Sun H. 2006. Pentacyclic triterpenes. Part 3: synthesis and biological evaluation of oleanolic acid derivatives as novel inhibitors of glycogen phosphorylase. Bioorg Med Chem Lett 16:2915–2919. doi: 10.1016/j.bmcl.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 27.Gururajanna B, Al-Katib AA, Li YW, Aranha O, Vaitkevicius VK, Sarkar FH. 1999. Molecular effects of taxol and caffeine on pancreatic cancer cells. Int J Mol Med 4:501–507. [DOI] [PubMed] [Google Scholar]
- 28.Jang MH, Shin MC, Kang IS, Baik HH, Cho YH, Chu JP, Kim EH, Kim CJ. 2002. Caffeine induces apoptosis in human neuroblastoma cell line SK-N-MC. J Korean Med Sci 17:674–678. doi: 10.3346/jkms.2002.17.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qi W, Qiao D, Martinez JD. 2002. Caffeine induces TP53-independent G1-phase arrest and apoptosis in human lung tumor cells in a dose-dependent manner. Radiat Res 157:166–174. doi: 10.1667/0033-7587(2002)157[0166:CITIGP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 30.Saiki S, Sasazawa Y, Imamichi Y, Kawajiri S, Fujimaki T, Tanida I, Kobayashi H, Sato F, Sato S, Ishikawa KI, Imoto M, Hattori N. 2011. Caffeine induces apoptosis by enhancement of autophagy via PI3K/Akt/mTOR/p70S6K inhibition. Autophagy 7:176–187. doi: 10.4161/auto.7.2.14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu DM, Zhao D, Li DZ, Xu DY, Chu WF, Wang XF. 2011. Maslinic acid induces apoptosis in salivary gland adenoid cystic carcinoma cells by Ca2+-evoked p38 signaling pathway. Naunyn-Schmiedebergs Arch Pharmacol 383:321–330. doi: 10.1007/s00210-011-0598-x. [DOI] [PubMed] [Google Scholar]
- 32.Reyes-Zurita FJ, Pachon-Pena G, Lizarraga D, Rufino-Palomares EE, Cascante M, Lupianez JA. 2011. The natural triterpene maslinic acid induces apoptosis in HT29 colon cancer cells by a JNK-p53-dependent mechanism. BMC Cancer 11:154. doi: 10.1186/1471-2407-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deponte M. 2008. Programmed cell death in protists. Biochim Biophys Acta 1783:1396–1405. doi: 10.1016/j.bbamcr.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 34.Martín-Navarro CM, López-Arencibia A, Sifaoui I, Reyes-Batlle M, Valladares B., Martinez-Carretero E, Piñero JE, Maciver SK, Lorenzo-Morales J. 2015. Statins and voriconazole induce programmed cell death in Acanthamoeba castellanii. Antimicrob Agents Chemother 59:2817–2824. doi: 10.1128/AAC.00066-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao LY, Kwaik YA. 2000. The mechanism of killing and exiting the protozoan host Acanthamoeba polyphaga by Legionella pneumophila. Environ Microbiol 2:79–90. doi: 10.1046/j.1462-2920.2000.00076.x. [DOI] [PubMed] [Google Scholar]
- 36.Feng Y, Hsiao YH, Chen HL, Chu CS, Tang P, Chiu CH. 2009. Apoptosis-like cell death induced by Salmonella in Acanthamoeba rhysodes. Genomics 94:132–137. doi: 10.1016/j.ygeno.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Nakisah MA, Ida Muryany MY, Fatimah H, Nor Fadilah R, Zalilawati MR, Khamsah S, Habsah M. 2012. Anti-amoebic properties of a Malaysian marine sponge Aaptos sp. on Acanthamoeba castellanii. World J Microbiol Biotechnol 28:1237–1244. doi: 10.1007/s11274-011-0927-8. [DOI] [PubMed] [Google Scholar]
- 38.Thrane C, Kaufmann U, Stummann BM, Olsson S. 2004. Activation of caspase-like activity and poly (ADP-ribose) polymerase degradation during sporulation in Aspergillus nidulans. Fungal Genet Biol 41:361–368. doi: 10.1016/j.fgb.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Silva RD, Sotoca R, Johansson B, Ludovico P, Sansonetty F, Silva MT, Peinado JM, Corte-Real M. 2005. Hyperosmotic stress induces metacaspase- and mitochondria-dependent apoptosis in Saccharomyces cerevisiae. Mol Microbiol 58:824–834. doi: 10.1111/j.1365-2958.2005.04868.x. [DOI] [PubMed] [Google Scholar]
- 40.Bozhkov PV, Filonova LH, Suarez MF. 2005. Programmed cell death in plant embryogenesis. Curr Top Dev Biol 67:135–179. doi: 10.1016/S0070-2153(05)67004-4. [DOI] [PubMed] [Google Scholar]
- 41.Trzyna WC, Legras XD, Cordingley JS. 2008. A type-1 metacaspase from Acanthamoeba castellanii. Microbiol Res 163:414–423. doi: 10.1016/j.micres.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 42.Saheb E, Trzyna W, Bush J. 2013. An Acanthamoeba castellanii metacaspase associates with the contractile vacuole and functions in osmoregulation. Exp Parasitol 133:314–326. doi: 10.1016/j.exppara.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Matz C, Webb JS, Schupp PJ, Phang SY, Penesyan A, Egan S, Steinberg P, Kjelleberg S. 2008. Marine biofilm bacteria evade eukaryotic predation by targeted chemical defense. PLoS One 3:e2744. doi: 10.1371/journal.pone.0002744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Proskuryakov SY, Konoplyannikov AG, Gabai VL. 2003. Necrosis: a specific form of programmed cell death? Exp Cell Res 283:1–16. doi: 10.1016/S0014-4827(02)00027-7. [DOI] [PubMed] [Google Scholar]
- 45.Sánchez-González M, Lozano-Mena G, Juan ME, García-Granados A, Planas JM. 2013. 2013. Assessment of the safety of maslinic acid, a bioactive compound from Olea europaea L. Mol Nutr Food Res 57:339–346. doi: 10.1002/mnfr.201200481. [DOI] [PubMed] [Google Scholar]
- 46.Sánchez-González M, Colom H, Lozano-Mena G, Juan ME, Planas JM. 2014. Population pharmacokinetics of maslinic acid, a triterpene from olives, after intravenous and oral administration in rats. Mol Nutr Food Res 58:1970–1979. doi: 10.1002/mnfr.201400147. [DOI] [PubMed] [Google Scholar]
- 47.Martín-Navarro CM, Lorenzo-Morales J, Cabrera-Serra MG, Rancel F, Coronado-Alvarez NM, Piñero JE, Valladares B. 2008. The potential pathogenicity of chlorhexidine-sensitive Acanthamoeba strains isolated from contact lens cases from asymptomatic individuals in Tenerife, Canary Islands, Spain. J Med Microbiol 57:1399–1404. doi: 10.1099/jmm.0.2008/003459-0. [DOI] [PubMed] [Google Scholar]
- 48.McBride J, Ingram PR, Henríquez FL, Roberts CW. 2005. Development of colorimetric microtiter plate assay for assessment of antimicrobials against Acanthamoeba. J Clin Microbiol 43:629–634. doi: 10.1128/JCM.43.2.629-634.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martín-Navarro CM, Lorenzo-Morales J, Machin RP, López-Arencibia A, García-Castellano JM, de Fuentes I, Loftus B, Maciver SK, Valladares B, Piñero JE. 2013. Inhibition of 3-hydroxy-3-methylglutaryl-coenzyme A reductase and application of statins as a novel effective therapeutic approach against Acanthamoeba infections. Antimicrob Agents Chemother 57(1):375–381. doi: 10.1128/AAC.01426-12. [DOI] [PMC free article] [PubMed] [Google Scholar]