Abstract
Irritable bowel syndrome (IBS) is a chronic functional disorder of the gastrointestinal tract and is one of the most commonly diagnosed gastrointestinal diseases. The impact of IBS on the general population is large due to its high prevalence, suboptimal medical treatments and significant economic burden. The pathophysiology of IBS is complex and treatments are often symptom-specific. The most common therapeutic approaches for IBS include education and reassurance, lifestyles (especially nutrition-based interventions), peripherally acting medications (which typically target motility), centrally acting medications (which target visceral hypersensitivity and pain) and psychological interventions (which aim to reduce the effects of stress or symptom-specific anxiety). A beneficial dietary approach might include the following measures: a diet low in fermentable oligo-,di- and monosaccharides and polyols (FODMAPs), limitation or exclusion of gas-producing foods and/or lactose and gluten and fiber supplementation in selected cases. New therapeutic agents, namely nutraceutics, are also an interesting option in the management of IBS patients. This paper will focus on available dietary interventions for IBS and will review the evidence for nutrition-based therapies.
Keywords: irritable bowel syndrome, functional gastrointestinal disorders, food, FODMAPs, intolerance, intestinal microbiota
Introduction
Irritable bowel syndrome (IBS) is a chronic functional gastrointestinal disorder (FGID) and is one of the most commonly diagnosed gastrointestinal diseases. The worldwide prevalence of IBS ranges from 10% to 20% [1,2] with an estimated incidence of 1.4–1.5% according to long follow-up studies lasting 10–12 years [3, 4]. IBS is more common in women and in young adults [5]. IBS is responsible for about one-third of all referrals to gastroenterology specialists and is associated with significant economic costs and psychosocial burden [6–8].
The recent ROME IV Consensus requires abdominal pain to be recurrent and associated with defecation or change in bowel habits (Table 1) [9]. IBS subtypes include constipation-predominant (IBS-C), diarrhea-predominant (IBS-D), and IBS with predominant irregular (mixed diarrhea/constipation) bowel habits (IBS-M). IBS subtypes are classified according to the 7-point Bristol Stool Form Scale, which is based on stool appearance ranging from type 1 (separate hard lumps like nuts, hard to pass) to type 7 (watery, no solid pieces, entirely liquid) and is considered a reliable marker for colonic transit [9–12]. IBS subtyping is more accurate when patients have at least 4 days of abnormal bowel habits monthly in the absence of any specific treatment such as laxatives or antidiarrheal agents using a diary report of at least 14 days. An alteration in stool consistency (i.e. hard/lumpy stools or loose/watery stools) for more than 25% of bowel movements is the threshold for classification in the various IBS subtypes.
Table 1.
Diagnostic criteria for irritable bowel syndrome (IBS) [9]
| Recurrent abdominal pain (on average at least 1 day per week in the last 3 months) associated with two or more of the following criteria |
|
| Criteria must be fulfilled for the last 3 months with symptom onset at least 6 months before diagnosis |
A number of pathophysiological abnormalities have been described in IBS patients [13–15] and include visceral hypersensitivity [16–19], motor abnormalities of the gastrointestinal tract [20–23], infectious gastroenteritis [24–27], intestinal inflammation and visceral hyperalgesia [28,29], genetic susceptibility [30,31] and psychosocial factors [32–34].
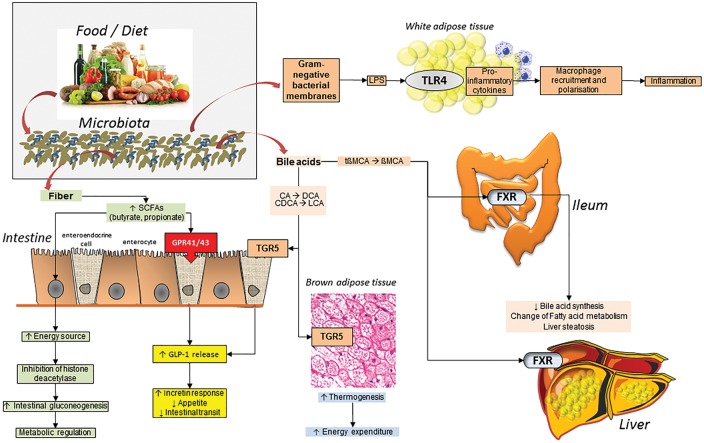
The intestinal microbiota plays a key role in metabolic, protective and structural functions [15,35–45] (Figure 1). The role of altered intestinal microbiota in IBS has been progressively investigated [46–50] (see also ref. [15] for extensive information) and is shown to undergo either qualitative, quantitative and/or locoregional changes. Such changes might heavily affect the fermentation capacity in IBS compared with healthy subjects [15,36,46–50]. Major abnormalities involve temporal instability [51,52], degree of variations [53,54] and microbiota composition according to IBS subtypes [47–50,52,55] and increased intestinal mucosal permeability associated with a low-grade of inflammation [15,51,56–58].
Figure 1.
Complex pathways linking diet to microbiota to fermentation and a number of metabolic processes in the body. Fermented dietary fiber results in short-chain fatty acids (SCFAs) production. In enterocytes, this process provides an energy source and is associated with histone deacetylase (HDAC) inhibition in enterocytes, stimulation of intestinal gluconeogenesis and metabolic regulation. SCFAs stimulate the G-protein coupled receptor 41 and 43 while the secondary bile acid lithocholic acid (LCA) and deoxycholic acid (DCA) stimulate the bile acid receptor TGR5 in the enteroendocrine cell with release of glucagon-like peptide-1 (GLP-1). This step, in turn, increases incretin secretion, suppresses appetite and reduces intestinal transit. Following microbiota biotransformation of primary into secondary bile salts, the stimulation of TGR5 in the brown adipose tissue promotes thermogenesis and energy expenditure. Also, the deconjugation of taurobetamuricholic acid (tbMCA) provides the repression of the natural farnesoid X receptor (FXR), decreased bile acid synthesis and changes of fatty acid (FA) metabolism. Gram-negative bacterial membranes produce lipopolysaccharide (LPS) a pro-inflammatory molecule that induces macrophage recruitment and polarization in white adipose tissue inducing inflammation through Toll-like receptor 4 (TLR4)
Adapted from: Arora T and Bäckhed F. The gut microbiota and metabolic disease: current understanding and future perspectives. J Intern Med 2016;280:339–49 [45].
Since the symptoms that define IBS are not unique, organic diseases that can mimic IBS (i.e. inflammatory bowel disease, celiac disease, intolerance for carbohydrates such as fructose and lactose, neoplasia, microscopic colitis etc.) must be excluded. The initial diagnosis of patients suspected to have IBS must therefore include a careful history, physical exam and limited diagnostic testing to evaluate for alarm features [59] that might require further evaluation. Alarm features include unexplainable or consistent weight loss, unexplained iron- deficiency anemia, rectal bleeding (in the absence of documented bleeding hemorrhoids or anal fissures), symptoms present at night, family history of colorectal cancer, celiac disease or inflammatory bowel disease, unexplainable fever and onset of symptoms after the age of 50 years. Limited laboratory studies include blood count (to rule out anemia or elevated white blood cell count) and, in patients with diarrhea, C-reactive protein (CRP), fecal calprotectin [60], serology for celiac disease and stool analysis for parasites. Further diagnostic tests to be considered on a case-by-case basis include colonoscopy and upper endoscopy with biopsies and H2-breath tests to rule out carbohydrate malabsorption [36,37,61].
After establishing a confident diagnosis of IBS, a consistent and therapeutic relationship must be established with the patient. This approach has been shown to improve patient outcomes [62–64]. A patient’s expectations must be clearly identified and, if possible, addressed [65]. Therapy is based on both type and severity of IBS symptoms.
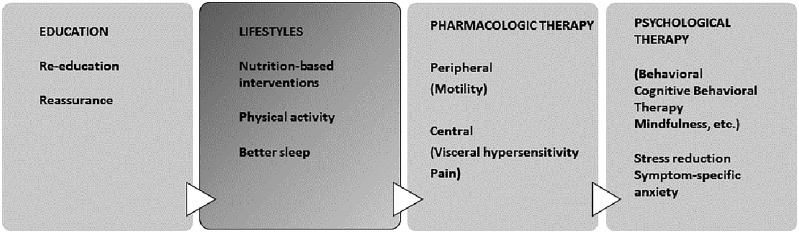
The most common therapeutic approaches for IBS include education and reassurance, lifestyles (especially nutrition-based interventions), peripherally acting medications (which typically target motility) and centrally acting medications (which target visceral hypersensitivity and pain) and psychological interventions (which aim to reduce the effects of stress or symptom-specific anxiety) [9,66] (Figure 2). This paper will focus on available dietary interventions for IBS and will review the evidence for nutrition-based therapies.
Figure 2.
General therapeutic approaches in irritable bowel syndrome (IBS) patients
Food Intolerance
Nutrition-based interventions are often recommended by primary care physicians and gastroenterologists, especially when patients report that their symptoms worsen after eating certain foods [67], a condition generally referred to as food “intolerance” [68–70]. Approximately 84% of patients report that their symptoms are triggered by at least one food item [68], and 62% report limiting their diet on their own without assistance from a gastroenterologist or nutritionist [69]. These food triggers do not reflect food allergies (as it would, for example, in celiac disease). A dietary interview is required to identify specific foods that may cause or aggravate IBS symptoms. Attention should be paid to the ingestion of wheat, dairy products, coffee, fruits, juices, vegetables, sweetened soft drinks and chewing gum.
Although the mechanisms of food intolerance in IBS remain unclear, there are currently three proposed pathways by which intolerance is hypothesized to develop: food hypersensitivity (immune-mediated); food chemicals (bioactive molecules) and luminal distension [71]. In the food hypersensitivity hypothesis, it is suggested that low-grade inflammation may occur in response to certain foods as a result of increased epithelial barrier permeability [72]. In the food chemicals hypothesis, attention is directed to salicylates, glutamates and amines, which may act directly on neural and mast cells [73]. This hypothesis has traditionally been tested through elimination diets and requires further research. Finally, luminal distension has been proposed as a mechanism for food intolerance in IBS in that certain molecules may increase water and gas volume, thereby causing bloating, pain and increased visceral hypersensitivity. The dietary factors discussed in this paper will each target at least one of these mechanisms.
Common Dietary Targets in Treating IBS
Fiber
Fiber can act as a bulking agent to improve intestinal transit and decrease constipation in a subgroup of IBS patients. Thus, dietary recommendations for IBS patients often include fiber supplementation, especially with soluble (psyllium/ispaghula husk) rather than insoluble (bran) fibers [59,74]. Psyllium/ispaghula should be started from low doses in order to avoid gas and abdominal bloating side-effects [75–77]. The optimal dose of fiber for IBS has not been established, but in general a target of 20–30 grams of total diet and supplementary fiber is reasonable. The evidence for fiber as a treatment for IBS is mixed. A systematic review based on 12 trials found no beneficial effect for bulking agents (either soluble fiber or insoluble fiber) over placebo for improving abdominal pain, global assessment or symptom scores [78]. Another meta-analysis on 12 trials found marginal improvement of symptoms with fiber (ispaghula husk) [79].
Gas-producing foods
IBS patients may also benefit from excluding gas-producing foods derived from fermentable substrates known to exacerbate symptoms. Foods associated with an increase in intestinal gas and flatulence include alcohol, apricots, bagels, bananas, beans, Brussels sprouts, caffeine, carrots, celery, onions, pretzels, prunes, raisins and wheat germ [15,36,80].
FODMAPs
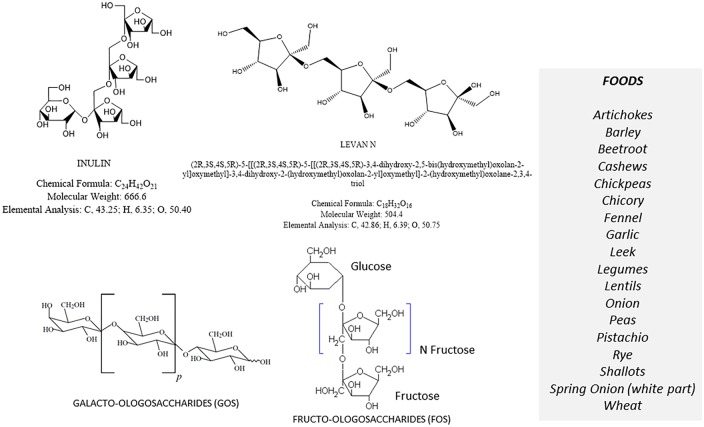
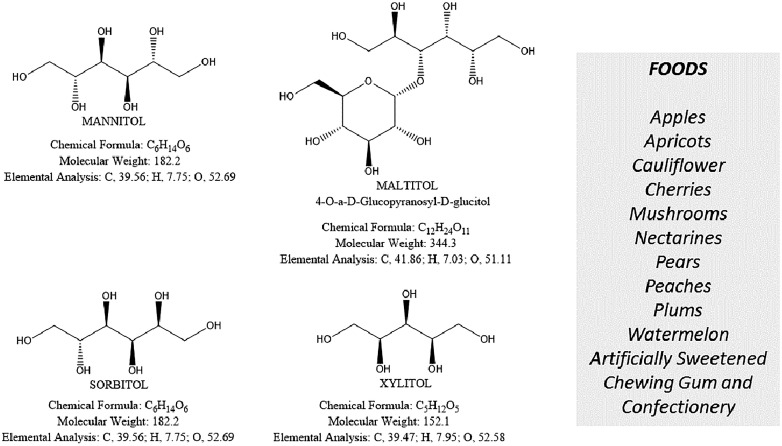
The acronym FODMAPs indicates common dietary Fermentable Oligo-, Di-, and Monosaccharides And Polyols, which includes fructans, galacto-oligosaccharides (Oligosaccharides), lactose (Disaccharide), fructose (Monosaccharide) especially in excess of glucose, mannitol, sorbitol, maltilol and xylitol (Polyols). The FODMAP family is composed of short-chain carbohydrates, which are poorly absorbed in the intestine. FODMAPs are contained in a large number of foods and are posited to influence IBS symptoms and other functional gastrointestinal diseases [81] (Figures 3–6).
Figure 3.
The fructans, galacto-oligosaccharides (FGO) and principal FGO lactose-containing foods
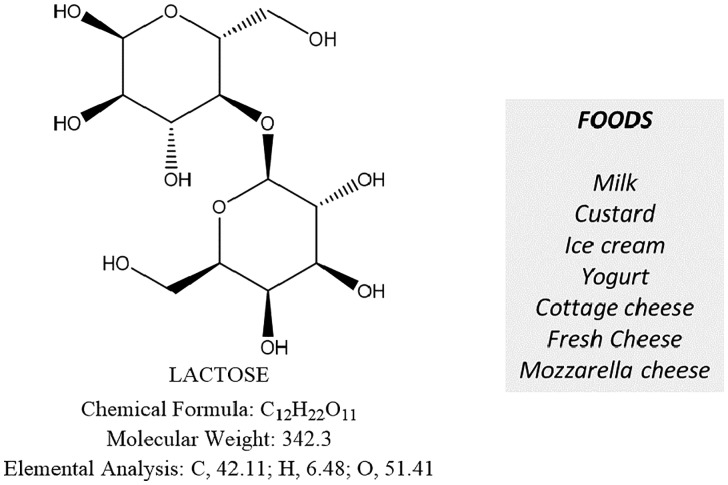
Figure 4.
The disaccharide lactose and principal lactose-containing foods
Figure 5.
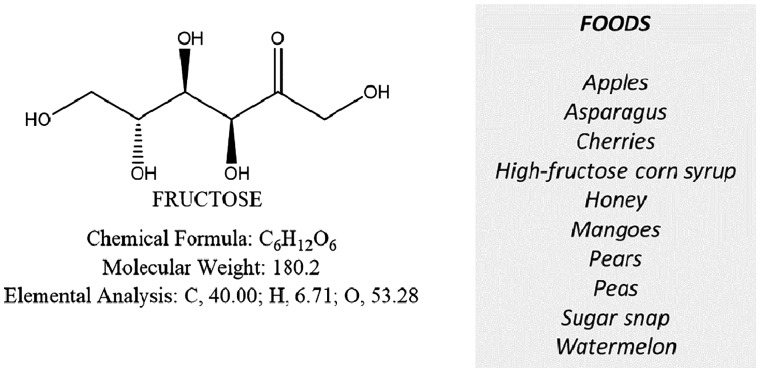
The monosaccharide fructose and principal fructose-containing foods
Figure 6.
Polyols and principal polyol-containing foods
FODMAPs behave as osmotically active molecules in the lumen of the small intestine and colon. The intestinal microbiota induces rapid fermentation of FODMAPs with production of hydrogen (H2) and methane. These gasses increase intraluminal tension and act on the intestinal wall causing symptoms such as abdominal bloating and pain [82]. Another consequence of increased FODMAP fermentation might include increases in intestinal permeability and (low-grade) inflammatory response [83]. In a randomized, controlled, single-blind crossover trial, 30 IBS patients who had not previously tried diets for their IBS reported improvement in overall gastrointestinal symptom scores compared with those on a standard Australian diet. While all IBS subtypes reported greater satisfaction with stool consistency, patients with IBS-D also reported improvement in their stool frequency [84]. A diet low in FODMAPs might therefore be worth trying in IBS patients [84,85], although long-term efficacy and safety, particularly on colonic health and microbiome, require further studies [75].
Other FODMAP-containing foods
In IBS patients, a diet low in fructose, fructans or a mixture of both improved symptoms [86]. Another study found that a “traditional IBS diet” (i.e regular meal pattern; avoidance of large meals and gas-producing foods such as beans, cabbage, onions and fat; reduced intake of insoluble fibers and caffeine) improved the symptoms of IBS after 4 weeks, similar to a low-FODMAP diet [87]. Overall, given the potential health benefits of many FODMAP-containing foods, less restrictive diets need to be considered; if a low FODMAP diet is pursued, patients should progressively reintroduce some FODMAP-containing foods, while maintaining the best healthy diet [36,81,88].
Lactose
Either genetic or acquired intestinal lactase deficiency is commonly found [89], and exaggerated fermentation of malabsorbed lactose by the intestinal microbiota may trigger or exacerbate IBS symptoms [90]. Although the final diagnosis of lactose intolerance requires the simultaneous evaluation of gastrointestinal symptoms and H2 levels in expired air by breath-testing with 25g lactose) [15,36,37,89,90], a trial of a lactose-free diet might be indicated in patients who do not improve after exclusion of gas-producing foods. For those patients with proven lactase deficiency, a diet poor in lactose [91,92] might be indicated. The use of oral lactases [61] before the ingestion of lactose-containing foods might also be useful for decreasing or even abolishing the symptoms.
Sucrose
Sucrose malabsorption is a relatively rare condition. The estimated prevalence in individuals of European descent ranges from 1:500 to 1:2000 [93,94]. The prevalence appears to higher in Alaskan Eskimos and native individuals from Greenland, where the prevalence has been reported to be as high as 10% [95]. Severe congenital sucrase-isomaltase deficiency (CSID) is due to a mutation in the sucrase-isomaltase gene that encodes sucrose-isomaltase, on chromosome 3q26. Severe CSID usually presents in early childhood; however, less severe forms may present in adulthood. The gold standard for diagnosis is the assessment of enzymatic activity from small intestinal biopsy. A positive H2-sucrose breath test or genetic testing can also support the diagnosis of sucrose malabsorption.
Fructose
Some foods and drinks (honey, high-fructose corn syrup, apples, mangoes, some pears and cherries, sweetened drinks, etc.) are enriched in the FODMAP fructose. Similar to lactose intolerance, a form of fructose intolerance also exists, particularly in the presence of excess fructose in foods [96]. A careful dietary survey should be taken in these patients [81], and a final diagnosis should be made (e.g. by means of H2-fructose breath test) [36]. According to Choi et al [97], about one-third of patients with suspected IBS had fructose intolerance, and their symptoms improved on a fructose-restricted diet. By contrast, noncompliance was associated with persistent symptoms. Patients with fructose intolerance, however, tend to conscientiously avoid a number of potentially fermentable and generally healthy foods [36]. A thoughtful re-education program with periodic follow-up is therefore necessary.
Gluten
Although the role of gluten in non-celiac IBS patients remains controversial, dietary restriction of gluten might be effective for decreasing IBS symptoms in some patients. The effect is particularly evident in IBS-D patients who report no improvement of bloating and flatulence when avoiding gas-producing foods or after starting a low FODMAP diet. Mechanisms of the impact of gluten in these patients might involve gut dysfunction, especially in patients with a genetic predisposition for celiac disease who do not show evidence of fully evolved disease [98]. Additionally, the gut barrier might be impaired in IBS-D patients [58]. Symptom improvement was shown in a double-blind, randomized, placebo-controlled rechallenge trial in 34 IBS patients without celiac disease (i.e. HLA-DQ2 and HLA-DQ8 negative or normal duodenal biopsies) and whose IBS symptoms were controlled on a gluten-free diet. Patients were randomized to receive either a small amount of gluten in the form of 2 bread slices plus 1 muffin per day or to continue a gluten-free diet. After 6 weeks, patients who were randomized to receive gluten had worse symptomatic control for overall symptoms, pain, bloating, stool consistency and tiredness as compared with patients who were randomized to receive no added gluten [99]. In another randomized controlled 4-week trial, 45 IBS-D patients were stratified for a gluten-containing or gluten-free diet. Patients using gluten exhibited global worsening of daily bowel movements, higher small bowel (not colonic) permeability as measured by the lactulose:mannitol ratio, decreased histologically proven expression of mRNA encoding tight junction proteins in the small bowel and rectosigmoid mucosa and cytokine production. Changes were more evident in patients with HLA-DQ2/8-positive genotype [58]. Interestingly, in a placebo-controlled, crossover rechallenge study conducted in 2013, the addition of a gluten-free diet to IBS patients already on a low FODMAP diet did not give additional benefit [100].
Nutraceutical products
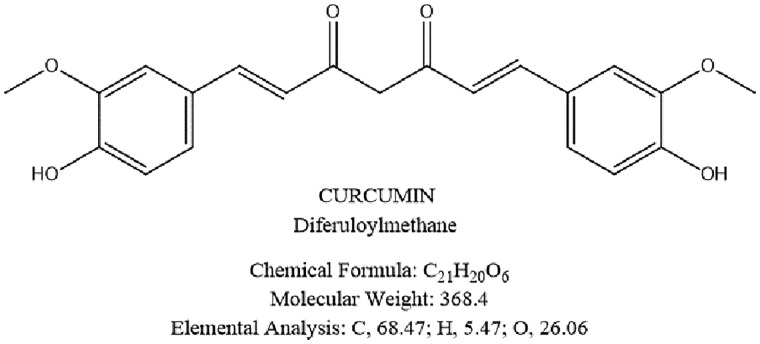
Curcumin is a phytochemical and a natural member of the Zingiberaceae ginger family that is derived from the rhizome (turmeric) of the indian herb Curcuma longa [101] (Figure 7). Curcumin has been used for centuries in both Ayurvedic and traditional Chinese medicine, targeting abdominal pain and bloating as well as inflammatory diseases (e.g. biliary disorders, rheumatism, sinusitis, injuries, fever) [102–105]. Of note, curcumin displays an anti-inflammatory activity in vitro, [105] and reduces mucosal injuries in the animal model of colitis [106–112]. Potential mechanisms of curcumin include the modulation of I-kappa B kinase activity, driven by inhibition of nuclear factor-κB (NF-kB) and pro-inflammatory cytokines (tumor necrosis factor alpha, interleukin 1β and 6) [113–116]. Several clinical studies also support a role for curcumin in both inflammatory bowel disorders and functional gastrointestinal diseases. In a randomized, double blind placebo-controlled study in patients with ulcerative colitis, curcumin (2 g/day) plus sulfasalazine or mesalamine for 6 months improved the Clinical Activity Index and the endoscopic score and prevented acute ulcerative colitis flares [117]. In a randomized controlled clinical trial, curcumin plus mesalazine induced remission in patients with mild-to-moderate ulcerative colitis [118]. In a small group of IBS patients [119], curcumin at 72 mg or 144 mg, given daily for 8 weeks, decreased abdominal pain intensity and improved quality of life.
Figure 7.
Chemical formula of curcumin
Anethole, the major component of fennel oil seeds, is chemically similar to the neurotransmitter dopamine and has a relaxant effect on intestinal smooth muscle, isolated rat uterus [120] and guinea pig trachea rings [121]. In a pilot study on IBS patients, the fennel reduced crampiform abdominal pain, a mechanism likely mediated by the anethole-dependent relaxation of intestinal smooth muscle [122]. The combination of curcumin-fennel essential oil has been recently used by our group and has improved symptoms and quality of life in IBS patients [123]. Further studies are required with longer follow-up and in subtypes of IBS.
Conclusions
The most recent guidelines on classification of IBS [9] confirm that the diagnosis of IBS and its clinical subtypes must rely on identifying typical symptoms and excluding alarm features that may suggest an organic disease. IBS is a heterogenous disorder with complex pathogenetic mechanisms contributing to the clinical features of IBS, which include abnormal intestinal motility and permeability, carbohydrate fermentation nutrient absorption, gas production and alterations in the intestinal microbiota. History-taking in IBS patients is important for ruling out the role of certain foods or other dietary components in the generation of symptoms.
The first-line approach to IBS includes dietary education while looking at foods responsible for the onset and worsening of symptoms. The avoidance, and progressive re-introduction of specific food components represents a subsequent step while evaluating the effects of gas-producing foods, lactose and fructose intolerance, other specific FODMAPs and gluten. The ultimate role of diet in different IBS subtypes needs further studies.
Acknowledgments
This work was partly supported by the Joint Programming Initiative “Healthy Diet for Healthy Life” (DEDIPAC) and funded by the Italian Ministry of Education, University and Research (MIUR).
Conflict of interest statement: none declared.
References
- 1.Longstreth GF, Thompson WG, Chey WD. et al. Functional bowel disorders. Gastroenterology 2006;130:1480–91. [DOI] [PubMed] [Google Scholar]
- 2.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol 2012;10:712–21.e4. [DOI] [PubMed] [Google Scholar]
- 3.Halder SL, Locke GR, 3rd, Schleck CD. et al. Natural history of functional gastrointestinal disorders: a 12-year longitudinal population-based study. Gastroenterology 2007;133:799–807. [DOI] [PubMed] [Google Scholar]
- 4.Ford AC, Forman D, Bailey AG. et al. Irritable bowel syndrome: a 10-yr natural history of symptoms and factors that influence consultation behavior. Am J Gastroenterol 2008;103:1229–39. [DOI] [PubMed] [Google Scholar]
- 5.Bharadwaj S, Barber MD, Graff LA, et al. Symptomatology of irritable bowel syndrome and inflammatory bowel disease during the menstrual cycle. Gastroenterol Rep (Oxf) 2015; 3:185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camilleri M, Heading RC, Thompson WG. Consensus report: clinical perspectives, mechanisms, diagnosis and management of irritable bowel syndrome. Aliment.Pharmacol Ther 2002;16:1407–30. [DOI] [PubMed] [Google Scholar]
- 7.Drossman DA, Camilleri M, Mayer EA. et al. AGA technical review on irritable bowel syndrome. Gastroenterology 2002;123:2108–31. [DOI] [PubMed] [Google Scholar]
- 8.Peery AF, Dellon ES, Lund J. et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012;143:1179–87.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mearin F, Lacy BE, Chang L. et al. Bowel Disorders. Gastroenterology 2016;150:1393–407.e5. [DOI] [PubMed] [Google Scholar]
- 10.Heaton KW, O'Donnell LJD. An Office Guide to Whole-Gut Transit Time. J Clin Gastroenterol 1994;19:28–30. [DOI] [PubMed] [Google Scholar]
- 11.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997;32:920–4. [DOI] [PubMed] [Google Scholar]
- 12.Patel P, Bercik P, Morgan DG. et al. Prevalence of organic disease at colonoscopy in patients with symptoms compatible with irritable bowel syndrome: cross-sectional survey. Scand J Gastroenterol 2015;50:816–23. [DOI] [PubMed] [Google Scholar]
- 13.Camilleri M, Lasch K, Zhou W. Irritable bowel syndrome: methods, mechanisms, and pathophysiology: the confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol 2012;303:G775–85. [DOI] [PubMed] [Google Scholar]
- 14.Camilleri M. Peripheral mechanisms in irritable bowel syndrome. N Engl J Med 2012;367:1626–35. [DOI] [PubMed] [Google Scholar]
- 15.Bonfrate L, Tack J, Grattagliano I. et al. Microbiota in health and irritable bowel syndrome: current knowledge, perspectives and therapeutic options. Scand J Gastroenterol 2013;48:995–1009. [DOI] [PubMed] [Google Scholar]
- 16.Whitehead WE, Holtkotter B, Enck P. et al. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology 1990;98:1187–92. [DOI] [PubMed] [Google Scholar]
- 17.Bouin M, Plourde V, Boivin M. et al. Rectal distention testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology 2002;122:1771–7. [DOI] [PubMed] [Google Scholar]
- 18.Zuo XL, Li YQ, Shi L. et al. Visceral hypersensitivity following cold water intake in subjects with irritable bowel syndrome. J Gastroenterol 2006;41:311–7. [DOI] [PubMed] [Google Scholar]
- 19.Nozu T, Kudaira M, Kitamori S. et al. Repetitive rectal painful distention induces rectal hypersensitivity in patients with irritable bowel syndrome. J Gastroenterol 2006;41:217–22. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt T, Hackelsberger N, Widmer R. et al. Ambulatory 24-hour jejunal motility in diarrhea-predominant irritable bowel syndrome. Scand J Gastroenterol 1996;31:581–9. [DOI] [PubMed] [Google Scholar]
- 21.Kumar D, Wingate D. Irritable bowel syndrome: a paroxysmal motor disorder. Lancet 1986;1:620–1. [DOI] [PubMed] [Google Scholar]
- 22.Simren M, Castedal M, Svedlund J. et al. Abnormal propagation pattern of duodenal pressure waves in the irritable bowel syndrome (IBS) [correction of (IBD)]. Dig Dis Sci 2000;45:2151–61. [DOI] [PubMed] [Google Scholar]
- 23.Portincasa P, Moschetta A, Baldassarre G. et al. Pan-enteric dysmotility, impaired quality of life and alexithymia in a large group of patients meeting the Rome II criteria for irritable bowel. World J Gastroenterol 2003;9:2293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall JK, Thabane M, Borgaonkar MR. et al. Postinfectious irritable bowel syndrome after a food-borne outbreak of acute gastroenteritis attributed to a viral pathogen. Clin Gastroenterol Hepatol 2007;5:457–60. [DOI] [PubMed] [Google Scholar]
- 25.Marshall JK, Thabane M, Garg AX. et al. Incidence and epidemiology of irritable bowel syndrome after a large waterborne outbreak of bacterial dysentery. Gastroenterology 2006;131:445–50. [DOI] [PubMed] [Google Scholar]
- 26.Thabane M, Kottachchi DT, Marshall JK. Systematic review and meta-analysis: The incidence and prognosis of post-infectious irritable bowel syndrome. Aliment Pharmacol Ther 2007;26:535–44. [DOI] [PubMed] [Google Scholar]
- 27.Halvorson HA, Schlett CD, Riddle MS. Postinfectious irritable bowel syndrome--a meta-analysis. Am J Gastroenterol 2006;101:1894–9. [DOI] [PubMed] [Google Scholar]
- 28.Chadwick VS, Chen W, Shu D. et al. Activation of the mucosal immune system in irritable bowel syndrome. Gastroenterology 2002;122:1778–83. [DOI] [PubMed] [Google Scholar]
- 29.Liebregts T, Adam B, Bredack C. et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology 2007;132:913–20. [DOI] [PubMed] [Google Scholar]
- 30.Saito YA, Petersen GM, Locke GR., 3rd et al. The genetics of irritable bowel syndrome. Clin Gastroenterol Hepatol 2005;3:1057–65. [DOI] [PubMed] [Google Scholar]
- 31.Beyder A, Mazzone A, Strege PR. et al. Loss-of-function of the voltage-gated sodium channel NaV1.5 (Channelopathies) in patients with irritable bowel syndrome. Gastroenterology 2014;146:1659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang L. The role of stress on physiological responses and clinical symptoms in irritable bowel syndrome. Gastroenterology 2011;140:761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drossman DA, McKee DC, Sandler RS. et al. Psychosocial factors in the irritable bowel syndrome. A multivariate study of patients and nonpatients with irritable bowel syndrome. Gastroenterology 1988;95:701–8. [DOI] [PubMed] [Google Scholar]
- 34.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 1997;11:395–402. [DOI] [PubMed] [Google Scholar]
- 35.Mans JJ, von Lackum K, Dorsey C. et al. The degree of microbiome complexity influences the epithelial response to infection. BMC Genomics 2009;10:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonfrate L, Krawczyk M, Lembo A. et al. Effects of dietary education, followed by a tailored fructose-restricted diet in adults with fructose malabsorption. Eur J Gastroenterol Hepatol 2015;27:785–96. [DOI] [PubMed] [Google Scholar]
- 37.Gasbarrini A, Corazza GR, Gasbarrini G. et al. Methodology and indications of H2-breath testing in gastrointestinal diseases: the Rome Consensus Conference. Aliment Pharmacol Ther 2009;29 Suppl 1:1–49. [DOI] [PubMed] [Google Scholar]
- 38.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res 2006;47:241–59. [DOI] [PubMed] [Google Scholar]
- 39.Lefebvre P, Cariou B, Lien F. et al. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev 2009;89:147–91. [DOI] [PubMed] [Google Scholar]
- 40.Begley M, Gahan CGM, Hill C. The interaction between bacteria and bile. FEMS Microbiol Rev 2005;29:625–51. [DOI] [PubMed] [Google Scholar]
- 41.Wong JMW, de Souza R, Kendall CWC. et al. Colonic health: Fermentation and short chain fatty acids. J Clin Gastroenterol 2006;40:235–43. [DOI] [PubMed] [Google Scholar]
- 42.Hijova E, Chmelarova A. Short chain fatty acids and colonic health. Bratisl Lek Listy 2007;108:354–8. [PubMed] [Google Scholar]
- 43.Chen WJ, Anderson JW, Jennings D. Propionate may mediate the hypocholesterolemic effects of certain soluble plant fibers in cholesterol-fed rats. Proc Exp Biol Med 1984;175:215–8. [DOI] [PubMed] [Google Scholar]
- 44.Lynch SV, Phimister EG, Pedersen O. The Human Intestinal Microbiome in Health and Disease. N Engl J Med 2016;375:2369–79. [DOI] [PubMed] [Google Scholar]
- 45.Arora T, Bäckhed F. The gut microbiota and metabolic disease: current understanding and future perspectives. J Intern Med 2016;280:339–49. [DOI] [PubMed] [Google Scholar]
- 46.Kassinen A, Krogius-Kurikka L, Makivuokko H. et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology 2007;133:24–33. [DOI] [PubMed] [Google Scholar]
- 47.Malinen E, Rinttila T, Kajander K. et al. Analysis of the fecal microbiota of irritable bowel syndrome patients and healthy controls with real-time PCR. Am J Gastroenterol 2005;100:373–82. [DOI] [PubMed] [Google Scholar]
- 48.Rajilic-Stojanovic M, Biagi E, Heilig HG. et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology 2011;141:1792–801. [DOI] [PubMed] [Google Scholar]
- 49.Saulnier DM, Riehle K, Mistretta TA. et al. Gastrointestinal microbiome signatures of pediatric patients with irritable bowel syndrome. Gastroenterology 2011;141:1782–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeffery IB, O'Toole PW, Ohman L. et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut 2012;61:997–1006. [DOI] [PubMed] [Google Scholar]
- 51.Matto J, Maunuksela L, Kajander K. et al. Composition and temporal stability of gastrointestinal microbiota in irritable bowel syndrome--a longitudinal study in IBS and control subjects. FEMS Immunol Med Microbiol 2005;43:213–22. [DOI] [PubMed] [Google Scholar]
- 52.Maukonen J, Satokari R, Mättö J. et al. Prevalence and temporal stability of selected clostridial groups in irritable bowel syndrome in relation to predominant faecal bacteria. J Med Microbiol 2006;55:625–33. [DOI] [PubMed] [Google Scholar]
- 53.Codling C, O’Mahony L, Shanahan F. et al. A molecular analysis of fecal and mucosal bacterial communities in irritable bowel syndrome. Dig Dis Sci 2010;55:392–7. [DOI] [PubMed] [Google Scholar]
- 54.Rajilić-Stojanović M, Smidt H, De Vos WM. Diversity of the human gastrointestinal tract microbiota revisited. Environ Microbiol 2007;9:2125–36. [DOI] [PubMed] [Google Scholar]
- 55.Lyra A, Rinttilä T, Nikkilä J. et al. Diarrhoea-predominant irritable bowel syndrome distinguishable by 16S rRNA gene phylotype quantification. World J Gastroenterol 2009;15:5936–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bischoff SC, Barbara G, Buurman W. et al. Intestinal permeability – a new target for disease prevention and therapy. BMC Gastroenterology 2014;14:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simren M, Barbara G, Flint HJ. et al. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut 2013;62:159–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vazquez-Roque MI, Camilleri M, Smyrk T. et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology 2013;144:903–11.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ford AC, Moayyedi P, Lacy BE. et al. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol 2014;109 Suppl 1:S2–26. [DOI] [PubMed] [Google Scholar]
- 60.Menees SB, Kurlander J, Goel A. et al. Sa1079 A Meta-Analysis of the Utility of Common Serum and Fecal Biomarkers in Adults With IBS. Gastroenterology 2014;146:S-194. [Google Scholar]
- 61.Portincasa P, Di Ciaula A, Vacca M. et al. Beneficial effects of oral tilactase on patients with hypolactasia. Eur J Clin Invest 2008;38:835–44. [DOI] [PubMed] [Google Scholar]
- 62.Kaptchuk TJ, Kelley JM, Conboy LA. et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ 2008;336:999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Drossman DA, Thompson WG. The irritable bowel syndrome: review and a graduated multicomponent treatment approach. Ann Intern Med 1992;116:1009–16. [DOI] [PubMed] [Google Scholar]
- 64.Owens DM, Nelson DK, Talley NJ. The irritable bowel syndrome: long-term prognosis and the physician-patient interaction. Ann Intern Med 1995;122:107–12. [DOI] [PubMed] [Google Scholar]
- 65.Halpert A, Dalton CB, Palsson O. et al. Irritable bowel syndrome patients' ideal expectations and recent experiences with healthcare providers: a national survey. Dig Dis Sci 2010;55:375–83. [DOI] [PubMed] [Google Scholar]
- 66.Johannesson E, Simren M, Strid H. et al. Physical activity improves symptoms in irritable bowel syndrome: a randomized controlled trial. Am J Gastroenterol 2011;106:915–22. [DOI] [PubMed] [Google Scholar]
- 67.Bijkerk CJ, de Wit NJ, Stalman WA. et al. Irritable bowel syndrome in primary care: the patients' and doctors' views on symptoms, etiology and management. Can J Gastroenterol 2003;17:363–8. [DOI] [PubMed] [Google Scholar]
- 68.Simren M, Mansson A, Langkilde AM. et al. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion 2001;63:108–15. [DOI] [PubMed] [Google Scholar]
- 69.Monsbakken KW, Vandvik PO, Farup PG. Perceived food intolerance in subjects with irritable bowel syndrome-- etiology, prevalence and consequences. Eur J Clin Nutr 2006;60:667–72. [DOI] [PubMed] [Google Scholar]
- 70.Atkinson W, Sheldon TA, Shaath N. et al. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomised controlled trial. Gut 2004;53:1459–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gibson PR. Food intolerance in functional bowel disorders. J Gastroenterol Hepatol 2011;26 Suppl 3:128–31. [DOI] [PubMed] [Google Scholar]
- 72.Dunlop SP, Hebden J, Campbell E. et al. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol 2006;101:1288–94. [DOI] [PubMed] [Google Scholar]
- 73.Raithel M, Baenkler HW, Naegel A. et al. Significance of salicylate intolerance in diseases of the lower gastrointestinal tract. J Physiol Pharmacol 2005;56 Suppl 5:89–102. [PubMed] [Google Scholar]
- 74.Bijkerk CJ, de Wit NJ, Muris JW. et al. Soluble or insoluble fibre in irritable bowel syndrome in primary care? Randomised placebo controlled trial. BMJ 2009;339:b3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rao SS, Yu S, Fedewa A. Systematic review: dietary fibre and FODMAP-restricted diet in the management of constipation and irritable bowel syndrome. Aliment Pharmacol Ther 2015;41:1256–70. [DOI] [PubMed] [Google Scholar]
- 76.Drossman DA, Whitehead WE, Camilleri M. Irritable bowel syndrome: a technical review for practice guideline development. Gastroenterology 1997;112:2120–37. [DOI] [PubMed] [Google Scholar]
- 77.Ford AC, Talley NJ. Irritable bowel syndrome. BMJ 2012;345:e5836. [DOI] [PubMed] [Google Scholar]
- 78.Ruepert L,, Quartero AO,, de Wit NJ. et al. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev 2011:CD003460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ford AC, Talley NJ, Spiegel BM. et al. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. BMJ 2008;337:a2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhu Y, Zheng X, Cong Y. et al. Bloating and distention in irritable bowel syndrome: the role of gas production and visceral sensation after lactose ingestion in a population with lactase deficiency. Am J Gastroenterol 2013;108:1516–25. [DOI] [PubMed] [Google Scholar]
- 81.Shepherd SJ, Lomer MC, Gibson PR. Short-chain carbohydrates and functional gastrointestinal disorders. Am J Gastroenterol 2013;108:707–17. [DOI] [PubMed] [Google Scholar]
- 82.Ong DK, Mitchell SB, Barrett JS. et al. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol 2010;25:1366–73. [DOI] [PubMed] [Google Scholar]
- 83.Gibson PR, Shepherd SJ. Personal view: food for thought--western lifestyle and susceptibility to Crohn's disease. The FODMAP hypothesis. Aliment Pharmacol Ther 2005;21:1399–409. [DOI] [PubMed] [Google Scholar]
- 84.Halmos EP, Power VA, Shepherd SJ. et al. A Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome. Gastroenterology 2014;146:67–75.e5. [DOI] [PubMed] [Google Scholar]
- 85.Moayyedi P, Quigley EM, Lacy BE. et al. The Effect of Dietary Intervention on Irritable Bowel Syndrome: A Systematic Review. Clin Transl Gastroenterol 2015;6:e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shepherd SJ, Parker FC, Muir JG. et al. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol 2008;6:765–71. [DOI] [PubMed] [Google Scholar]
- 87.Bohn L, Storsrud S, Liljebo T. et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology 2015;149:1399–407.e2. [DOI] [PubMed] [Google Scholar]
- 88.McKenzie YA, Alder A, Anderson W. et al. British Dietetic Association evidence-based guidelines for the dietary management of irritable bowel syndrome in adults. J Hum Nutr Diet 2012;25:260–74. [DOI] [PubMed] [Google Scholar]
- 89.Krawczyk M, Wolska M, Schwartz S. et al. Concordance of genetic and breath tests for lactose intolerance in a tertiary referral centre. J Gastrointestin Liver Dis 2008;17:135–9. [PubMed] [Google Scholar]
- 90.Yang J, Deng Y, Chu H. et al. Prevalence and presentation of lactose intolerance and effects on dairy product intake in healthy subjects and patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2013;11:262–8.e1. [DOI] [PubMed] [Google Scholar]
- 91.American College of Gastroenterology Task Force on Irritable Bowel Syndrome; Brandt LJ, Chey WD, Foxx-Orenstein AE. et al. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol 2009;104 Suppl 1:S1–35. [DOI] [PubMed] [Google Scholar]
- 92.Bohmer CJ, Tuynman HA. The effect of a lactose-restricted diet in patients with a positive lactose tolerance test, earlier diagnosed as irritable bowel syndrome: a 5-year follow-up study. Eur J Gastroenterol Hepatol 2001;13:941–4. [DOI] [PubMed] [Google Scholar]
- 93.Gray GM, Conklin KA, Townley RR. Sucrase-isomaltase deficiency. Absence of an inactive enzyme variant. N Engl J Med 1976;294:750-3. [DOI] [PubMed] [Google Scholar]
- 94.Peterson ML, Herber R. Intestinal sucrase deficiency. Trans Assoc Am Physicians 1967;80:275–83. [PubMed] [Google Scholar]
- 95.Ament ME, Perera DR, Esther LJ. Sucrase-isomaltase deficiency-a frequently misdiagnosed disease. J Pediatr 1973;83:721–7. [DOI] [PubMed] [Google Scholar]
- 96.Choi YK, Johlin FC, Jr, Summers RW. et al. Fructose intolerance: an under-recognized problem. Am J Gastroenterol 2003;98:1348–53. [DOI] [PubMed] [Google Scholar]
- 97.Choi YK, Kraft N, Zimmerman B. et al. Fructose intolerance in IBS and utility of fructose-restricted diet. J Clin Gastroenterol 2008;42:233–8. [DOI] [PubMed] [Google Scholar]
- 98.Verdu EF, Huang X, Natividad J. et al. Gliadin-dependent neuromuscular and epithelial secretory responses in gluten-sensitive HLA-DQ8 transgenic mice. Am J Physiol Gastrointest Liver Physiol 2008;294:G217–25. [DOI] [PubMed] [Google Scholar]
- 99.Biesiekierski JR, Newnham ED, Irving PM. et al. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am J Gastroenterol 2011;106:508–14. [DOI] [PubMed] [Google Scholar]
- 100.Biesiekierski JR, Peters SL, Newnham ED. et al. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology 2013;145:320–8.e1–3. [DOI] [PubMed] [Google Scholar]
- 101.Gupta SC, Kismali G, Aggarwal BB. Curcumin, a component of turmeric: from farm to pharmacy. BioFactors 2013;39:2–13. [DOI] [PubMed] [Google Scholar]
- 102.Ammon HP, Wahl MA. Pharmacology of curcuma longa. Planta Med 1991;57:1–7. [DOI] [PubMed] [Google Scholar]
- 103.Ammon HP, Safayhi H, Mack T. et al. Mechanism of antiinflammatory actions of curcumine and boswellic acids. J Ethnopharmacol 1993;38:113–9. [DOI] [PubMed] [Google Scholar]
- 104.Patwardhan B, Warude D, Pushpangadan P. et al. Ayurveda and traditional Chinese medicine: a comparative overview. Evid Based Complement Alternat Med 2005;2:465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shishodia S, Sethi G, Aggarwal BB. Curcumin: getting back to the roots. Ann N Y Acad Sci 2005;1056:206–17. [DOI] [PubMed] [Google Scholar]
- 106.Lubbad A, Oriowo MA, Khan I. Curcumin attenuates inflammation through inhibition of TLR-4 receptor in experimental colitis. Mol Cell Biochem 2009;322:127–35. [DOI] [PubMed] [Google Scholar]
- 107.Billerey-Larmonier C, Uno JK, Larmonier N. et al. Protective effects of dietary curcumin in mouse model of chemically induced colitis are strain dependent. Inflamm Bowel Dis 2008;14:780–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang M, Deng CS, Zheng JJ. et al. Curcumin regulated shift from Th1 to Th2 in trinitrobenzene sulphonic acid-induced chronic colitis. Acta Pharmacol Sin 2006;27:1071–7. [DOI] [PubMed] [Google Scholar]
- 109.Martelli L, Ragazzi E, di Mario F. et al. A potential role for the vanilloid receptor TRPV1 in the therapeutic effect of curcumin in dinitrobenzene sulphonic acid-induced colitis in mice. Neurogastroenterol Motil 2007;19:668–74. [DOI] [PubMed] [Google Scholar]
- 110.Deguchi Y, Andoh A, Inatomi O. et al. Curcumin prevents the development of dextran sulfate Sodium (DSS)-induced experimental colitis. Dig Dis Sci 2007;52:2993–8. [DOI] [PubMed] [Google Scholar]
- 111.Ukil A, Maity S, Karmakar S. et al. Curcumin, the major component of food flavour turmeric, reduces mucosal injury in trinitrobenzene sulphonic acid-induced colitis. Br J Pharmacol 2003;139:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sugimoto K, Hanai H, Tozawa K. et al. Curcumin prevents and ameliorates trinitrobenzene sulfonic acid-induced colitis in mice. Gastroenterology 2002;123:1912–22. [DOI] [PubMed] [Google Scholar]
- 113.Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J 2013;15:195–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Epstein J, Docena G, MacDonald TT. et al. Curcumin suppresses p38 mitogen-activated protein kinase activation, reduces IL-1beta and matrix metalloproteinase-3 and enhances IL-10 in the mucosa of children and adults with inflammatory bowel disease. Br J Nutr 2010;103:824–32. [DOI] [PubMed] [Google Scholar]
- 115.Larmonier CB, Midura-Kiela MT, Ramalingam R. et al. Modulation of neutrophil motility by curcumin: implications for inflammatory bowel disease. Inflamm Bowel Dis 2011;17:503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jobin C, Bradham CA, Russo MP. et al. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J Immunol 1999;163:3474–83. [PubMed] [Google Scholar]
- 117.Hanai H, Iida T, Takeuchi K. et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clin Gastroenterol Hepatol 2006;4:1502–6. [DOI] [PubMed] [Google Scholar]
- 118.Lang A, Salomon N, Wu JC. et al. Curcumin in Combination With Mesalamine Induces Remission in Patients With Mild-to-Moderate Ulcerative Colitis in a Randomized Controlled Trial. Clin Gastroenterol Hepatol 2015;13:1444–9.e1. [DOI] [PubMed] [Google Scholar]
- 119.Bundy R, Walker AF, Middleton RW. et al. Turmeric extract may improve irritable bowel syndrome symptomology in otherwise healthy adults: a pilot study. J Altern Complement Med 2004;10:1015–8. [DOI] [PubMed] [Google Scholar]
- 120.Ostad SN, Soodi M, Shariffzadeh M. et al. The effect of fennel essential oil on uterine contraction as a model for dysmenorrhea, pharmacology and toxicology study. J Ethnopharmacol 2001;76:299–304. [DOI] [PubMed] [Google Scholar]
- 121.Boskabady MH, Khatami A, Nazari A. Possible mechanism(s) for relaxant effects of Foeniculum vulgare on guinea pig tracheal chains. Pharmazie 2004;59:561–-4. [PubMed] [Google Scholar]
- 122.Amjad H, Jafary HA. Foeniculum vulgare therapy in irritable bowel syndrome. Am J Gastroenterol 2000;95:2491. [Google Scholar]
- 123.Portincasa P, Bonfrate L, Scribano ML. et al. Curcumin and Fennel Essential Oil Improve Symptoms and Quality of Life in Patients with Irritable Bowel Syndrome. J Gastrointestin Liver Dis 2016;25:151–7. [DOI] [PubMed] [Google Scholar]