Fig. 2.
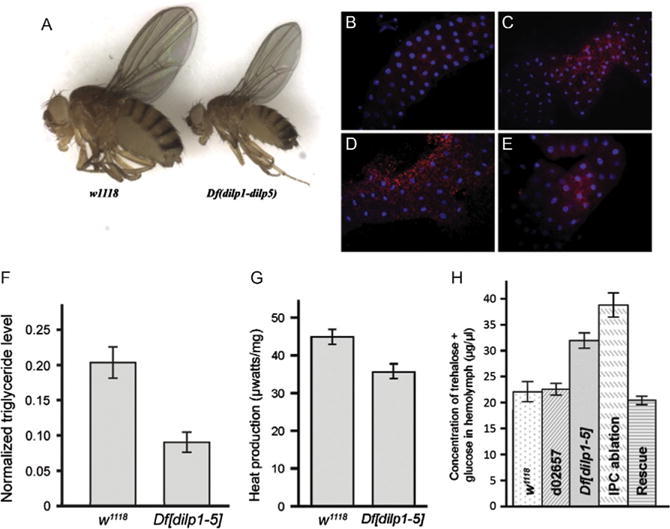
Deletion of dilps 1-5 generates flies with features of T1D. (A) A genomic deletion that removes genes encoding DILPs 1-5 (Df[dilp1-5]) is homozygous viable but flies are developmentally delayed and small. (B–E) Df[dilp1-5] homozygotes induce fat body autophagy while actively eating. Lysotracker staining (red) of dissected fat bodies from third-instar larvae. Hoechst 33342 (blue) reveals nuclei. For B, C, and E, larvae were actively eating, burrowed in the food, and had full guts. Genotypes: (B) Control parental line d02657;(C) Df[dilp1-5]/Df[dilp1-5]; (D) Control parental line d02657;third-instar larva removed from food and starved for 3 h in the presence of water (E) hsGAL4>UASdilp2; Df [dilp1-5]/Df[dilp1-5]. Feeding Df[dilp1-5] homozygotes (C) resembled starved control animals (D). (F) Total body triglyceride levels are lower in Df[dilp1-5] homozygotes. Total body triglyceride level and total body protein from w1118 or Df[dilp1-5] adult male flies were measured; data shows the triglyceride levels normalized to the total protein level. Error bars indicate standard error. (G) Overall metabolic activity was reduced in Df[dilp1-5] homozygotes. (H) Circulating sugar levels are elevated in Df[dilp1-5] homozygotes. Sugar levels (trehalose + glucose) were determined in hemolymph extracted from early third-instar larvae. Levels are indicated for negative controls: w1118 and parental line d02657;for experimental samples: Df[dilp1-5]/Df[dilp1-5], IPC-ablated animals, dilp2-GAL4>UAS-rpr, and rescued hsGAL4>UASdilp2; Df[dilp1-5]/Df[dilp1-5] animals. Larval hemolymph was collected from 10 to 15 animals and pooled for each genotype. The circulating sugar levels of Df[dilp1-5] homozygotes were higher than controls in early and late third-instar larvae (not shown). Levels were lowered by ubiquitous expression of DILP2. Sugar levels were highest in animals in which IPCs had been ablated (dilp2GAL4>UASrpr). Error bars indicate standard error. From Zhang, H., Liu, J., Li, C. R., Momen, B., Kohanski, R. A., & Pick, L. (2009). Deletion of Drosophila insulin-like peptides causes growth defects and metabolic abnormalities. Proceedings of the National Academy of Sciences of the United States of America, 106(46), 19617—19622.
