Abstract
The efficacy of 5-fluorouracil-based adjuvant chemotherapy differs according to poorly differentiated cluster grade in colorectal cancer. Poorly differentiated cluster may have an important role in determining the most suitable treatment strategy.
Keywords: poorly differentiated cluster, colorectal cancer, adjuvant chemotherapy
Abstract
Objective
Although poorly differentiated cluster has been reported to be a useful grading system for predicting prognosis in colorectal cancer, its relationship to chemotherapy efficacy has not been demonstrated. We aimed to investigate the association between poorly differentiated cluster and the efficacy of 5-fluorouracil-based adjuvant chemotherapy in stage III colorectal cancer.
Methods
This retrospective study enrolled 131 patients with stage III colorectal cancer who underwent curative resection: 72 received 5-fluorouracil-based adjuvant chemotherapy (chemotherapy group) and 59 did not (surgery-alone group). Poorly differentiated cluster was defined as a cancer cluster of ≥5 cancer cells without gland-like structure, and was classified into poorly differentiated cluster G1, G2 and G3 according to the number of clusters. The benefit of 5-fluorouracil-based adjuvant chemotherapy was evaluated based on poorly differentiated cluster grade.
Results
Thirty-nine, 40 and 52 patients were classified as poorly differentiated cluster G1, G2 and G3, respectively. Significant differences in the 5-year cumulative recurrence rate and relapse-free survival were observed between poorly differentiated cluster G1/G2 and G3 (26.7% vs. 47.5%, P = 0.010; 66.0% vs. 43.9%, P = 0.004). A comparison of cumulative recurrence rate and relapse-free survival between the chemotherapy and surgery-alone groups showed a significant benefit of adjuvant chemotherapy in poorly differentiated cluster G1/G2 patients (cumulative recurrence rate: 17.4% vs. 37.3%, P = 0.035; relapse-free survival: 79.5% vs. 51.9%, P = 0.002), but not in poorly differentiated cluster G3 patients (cumulative recurrence rate: 48.6% vs. 44.8%, P = 0.885; relapse-free survival: 51.4% vs. 32.7%, P = 0.068).
Conclusions
In stage III colorectal cancer, poorly differentiated cluster G1/G2 predicts a significant benefit from 5-fluorouracil-based adjuvant chemotherapy, whereas poorly differentiated cluster G3 predicts a poor response to it.
Introduction
In developed countries, colorectal cancer (CRC) is the second most common malignancy (1). Despite curative surgery, nodal metastatic disease still leads to death in ~30% of cases (2). Since the 1990s, adjuvant chemotherapy has been administered to decrease the risk of tumor recurrence and improve survival in CRC (3). It is well established that adjuvant therapy with 5-fluorouracil (5-FU) improves disease-free survival by 10–15% in stage III colon cancer (4–6) plus an additional 4–6% with 5-FU-based regimen plus oxaliplatin (oxaliplatin-based regimen) (7–9). Although TNM staging remains the most important determinant of CRC prognosis and treatment including adjuvant chemotherapy, there are other independent prognostic factors in addition to TNM staging.
Histopathological grading is one of the prognostic factors for CRC, independent of TNM stage (10–12). The most widely accepted histopathological grading is based on the degree of tumor differentiation. When a carcinoma has heterogeneity in differentiation, histopathological grading is determined based on the least differentiated component, not including the advancing edge of the tumor (13). Multivariate analysis has shown that histopathological grading of tumor differentiation is a TNM stage-independent prognostic factor, but there is significant interobserver variability (14–16).
Recently, poorly differentiated cluster (PDC) was reported to be a useful grading system for predicting prognosis in CRC patients (17). According to the original definition, PDC is composed of five or more cancer cells with no gland formation, and is found at the advancing edge of the tumor (18). On the basis of the count of PDCs, PDC was classified as grade (G) G1, G2 and G3, respectively (18). PDC affects the outcome independent of T and N categories (18), and in a recent multicenter study analyzing 3243 CRC patients, the quantification of PDCs to grade tumors was expected to be more objective than conventional histopathological grading and more informative for predicting prognosis than TNM staging (17).
The prognosis of CRC patients with PDC G3 is worse than those with PDC G1 or G2 (17,18). We hypothesized that tumors with PDC G3 are more tolerant of adjuvant chemotherapy compared with those with PDC G1 or G2. The aim of this study was to investigate the association between PDC grade and the efficacy of 5-FU-based adjuvant chemotherapy in stage III CRC.
Patients and methods
Patients
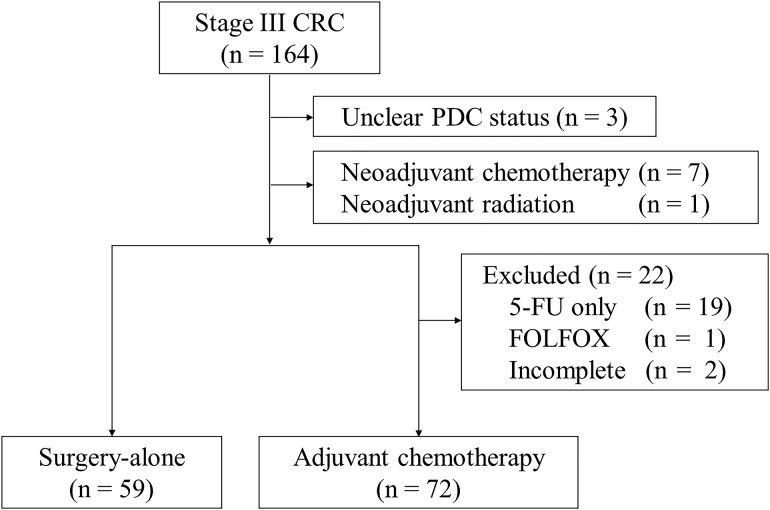
The institutional review board approved and issued a waiver of informed consent for this retrospective study. A total of 164 patients diagnosed with stage III CRC according to the AJCC seventh edition staging classification (19) who had curative surgery between 2000 and 2010 at Niigata University Medical and Dental Hospital, Niigata, Japan, were identified. We selected patients from our colorectal database using the following inclusion and exclusion criteria (Fig. 1): (i) patients diagnosed with adenocarcinoma were included, while (ii) patients who received endoscopic mucosal resection before operation (unclear PDC status), (iii) patients who received neoadjuvant therapy, (iv) patients who received adjuvant chemotherapy with ‘5-FU only’ or ‘oxaliplatin-based regimen’ and (v) patients who withdrew from adjuvant chemotherapy before completion were excluded. According to the criteria, 131 of 164 patients were included for further investigation. Among the 131 patients, 72 underwent adjuvant chemotherapy (chemotherapy group) and 59 did not because of age, comorbidity and/or patient preference (surgery-alone group). Among the 131 patients, the median follow-up period was 61 (1–150) months.
Figure 1.
Flow chart diagram of inclusion and exclusion criteria in this study.
CRC, colorectal cancer; PDC, poorly differentiated cluster.
’5-FU-based’ regimens
In this study, we classified adjuvant chemotherapy into three categories: ‘5-FU only’, ‘5-FU-based’ or ‘oxaliplatin-based’ regimens. ‘5-FU only’ refers to oral 5-FU drugs alone, such as oral tegafur–uracil (UFT). ‘Oxaliplatin-based’ regimen refers to a 5-FU-based plus oxaliplatin regimen, such as FOLFOX. ‘5-FU-based’ regimens included the Roswell Park Memorial Institute (RPMI) (20), oral UFT/leucovorin (LV) (21,22), capecitabine (4) and tegafur–gimeracil–oteracil potassium (S-1) regimens (23). The RPMI regimen comprised one cycle of 600 mg/m2 5-FU and 250 mg/m2 LV weekly for 6 weeks, with cycles repeated every 8 weeks for three cycles (20). The UFT/LV regimen comprised one cycle of 300 mg/m2/day UFT and 75 mg/day LV for 28 consecutive days, with cycles repeated every 5 weeks for five cycles (21,22). The capecitabine regimen comprised one cycle of 2500 mg/m2 capecitabine for 14 consecutive days, with cycles repeated every 3 weeks for eight cycles (4). The S-1 regimen comprised one cycle of S-1 (80 mg/day with body surface area [BSA] <1.25 m2, 100 mg/day with BSA 1.25–1.50 m2, 120 mg/day with BSA >1.50 m2) for 28 consecutive days, with cycles repeated every 6 weeks for four cycles (23). The choice of regimen was based on each physician's preference.
In this study, we focused on the association between PDC G3 and the ‘5-FU-based’ regimen, and excluded patients who received the ‘5-FU only’ or ‘oxaliplatin-based’ regimen. We excluded patients who received the ‘5-FU only’ regimen because there is little evidence for the effectiveness of this regimen (24). If we include this regimen, we may underestimate the efficacy of ‘5-FU-based’ adjuvant chemotherapy in this study. We also excluded patients who received the ‘oxaliplatin-based’ regimen to avoid the additive effect of oxaliplatin. We speculate that the mechanism of oxaliplatin resistance may be different from that of 5-FU resistance (25).
Definitions of PDC and histopathological grading
PDC was defined as cancer clusters in the stroma composed of ≥5 cancer cells that lack a gland-like structure (17). To quantify PDCs, the entire tumor including its advancing edge was first viewed at low-power magnification to identify the area containing the greatest number of PDCs. The clusters were then counted under a microscope using a ×20 objective lens (Fig. 2). Tumors with <5, 5–9 and ≥10 clusters were classified as G1, G2 and G3, respectively (18). With regard to assessment for mucinous carcinoma, malignant clusters with the above-mentioned features infiltrating the stroma with minimal extracellular mucin formation were classified as PDCs (17). In contrast, cancer cell clusters within a large mucin pool (i.e. mucinous lake) were not classified as PDCs (17). On the other hand, histopathological grading was determined on the basis of the least differentiated component, and the invading edge was regarded as suboptimal to evaluate histopathological grade according to the World Health Organization (WHO) classification (13). Two independent surgical pathologists (Y.S. and T.O.) blinded to all clinical details assessed each section. Any differences in assessment between the surgical pathologists were resolved by a double review using a multi-head microscope.
Figure 2.
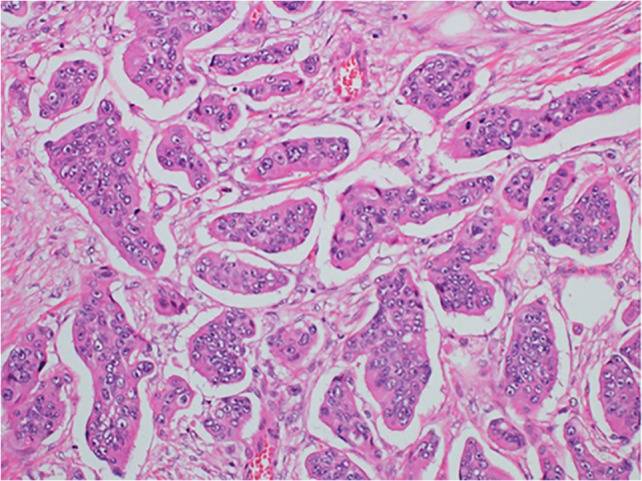
Poorly differentiated clusters (PDCs). Cancer cell clusters located in the stroma, comprising ≥5 cancer cells and lacking glandular formation are classified as PDCs. H&E staining, ×20 objective lens.
Prognostic factors
In this study, we assessed the association between PDC and the efficacy of 5-FU-based adjuvant chemotherapy by using cumulative recurrence rate (CRR) and relapse-free survival (RFS). To elucidate factors influencing CRR and RFS, 11 clinicopathological variables were tested in all 131 patients: age (<65 vs. ≥65 years), sex, American Society of Anesthesiologists physical status (ASA-PS; 1–2 vs. 3–4), tumor location (colon vs. rectum), tumor size (<50 vs. ≥50 mm), T category (T1–T3 vs. T4), histopathological grading (G1, G2 vs. G3), lymphatic invasion (absence vs. presence), venous invasion (absence vs. presence), N category (N1 vs. N2), PDC (G1, G2 vs. G3) and adjuvant chemotherapy (absence vs. presence).
Statistical analyses
Statistical analyses were performed with IBM SPSS Statistics 22 (IBM Japan, Inc., Tokyo, Japan). The relationships between each clinicopathological variable and PDC (G1, G2 vs. G3) were analyzed using Fisher's exact test. CRR and RFS were estimated using the Kaplan–Meier method. The log-rank test was used to assess significant differences between the subgroups by univariate analysis. P values <0.05 were considered statistically significant. To assess the potential prognostic factors for CRR and RFS, those with P values <0.05 in the univariate analyses were entered into the multivariate analysis. We used the Cox proportional hazards regression model to identify factors that were independently associated with CRR and RFS after surgery. In the PDC G1/G2 and G3 groups, the efficacy of adjuvant chemotherapy was evaluated by comparing CRR and RFS between the surgery-alone group and the chemotherapy group.
Results
Tumor grading based on PDCs and other clinicopathological characteristics
According to the number of PDCs, 39, 40 and 52 tumors were classified as G1, G2 and G3, respectively. Compared with PDC G1/G2, PDC G3 was significantly associated with histopathological grading G3 (P = 0.004), while there were no significant associations between PDCs and other clinicopathological characteristics (Table 1).
Table 1.
Association between poorly differentiated cluster and other clinicopathological characteristics
| PDC | P value | ||
|---|---|---|---|
| G1/G2 | G3 | ||
| (n = 79) | (n = 52) | ||
| Age | |||
| <65 | 30 | 21 | 0.855 |
| ≥65 | 49 | 31 | |
| Sex | |||
| Male | 49 | 29 | 0.585 |
| Female | 30 | 23 | |
| ASA-PS | |||
| 1/2 | 66 | 48 | 0.331 |
| 3/4 | 13 | 4 | |
| Tumor location | |||
| Colon | 31 | 18 | 0.712 |
| Rectum | 48 | 34 | |
| Tumor size (mm) | |||
| <50 | 39 | 23 | 0.595 |
| ≥50 | 40 | 29 | |
| Tumor stage | |||
| T1–T3 | 66 | 43 | 0.999 |
| T4 | 13 | 9 | |
| Histopathological grading | |||
| G1/G2 | 74 | 39 | 0.004 |
| G3 | 5 | 13 | |
| Lymphatic invasion | |||
| Absence | 39 | 19 | 0.156 |
| Presence | 40 | 33 | |
| Venous invasion | |||
| Absence | 39 | 27 | 0.859 |
| Presence | 40 | 25 | |
| Nodal involvement | |||
| N1 | 60 | 32 | 0.083 |
| N2 | 19 | 20 | |
| Adjuvant chemotherapy | |||
| Absence | 38 | 21 | 0.473 |
| Presence | 41 | 31 | |
PDC, poorly differentiated cluster; ASA-PS, American Society of Anesthesiologists physical status.
Clinical significance of PDC grade
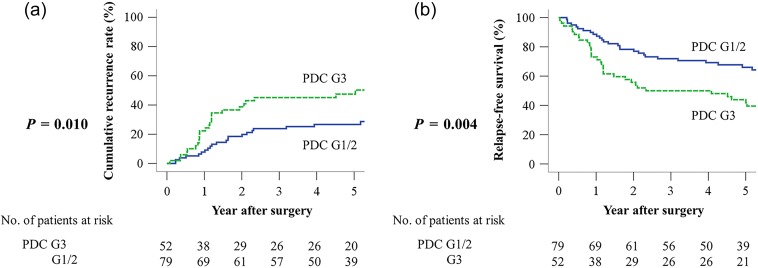
Significant differences were observed between PDC G1/G2 and G3 in the 5-year CRR (26.7% vs. 47.5%, P = 0.010) (Fig. 3a) and RFS (66.0% vs. 43.9%, P = 0.004) (Fig. 3b). Multivariate analysis identified that PDC G3 was an independent prognostic factor for CRR [hazard ratio (HR): 1.85, 95% confidence interval (CI): 1.01–3.38, P = 0.048] (Table 2) and RFS (HR: 2.00, 95% CI: 1.20–3.33, P = 0.008) (Table 3).
Figure 3.
Comparative cumulative recurrence rate (CRR) and relapse-free survival (RFS) curves of PDC G1/G2 and G3 groups in stage III CRC. (a) CRR and (b) RFS.
Table 2.
Univariate and multivariate analyses of prognostic factors for cumulative recurrence rate
| Variable | Modality | n | Univariate | Multivariate | ||
|---|---|---|---|---|---|---|
| 5-y CRR (%) | P value | HR (95% CI) | P value | |||
| Age (years) | <65 | 51 | 34.0 | 0.853 | ||
| ≥65 | 80 | 35.3 | ||||
| Sex | Male | 78 | 32.8 | 0.593 | ||
| Female | 53 | 37.9 | ||||
| ASA-PS | 1/2 | 114 | 33.5 | 0.565 | ||
| 3/4 | 17 | 44.4 | ||||
| Tumor location | Colon | 49 | 27.5 | 0.105 | ||
| Rectum | 82 | 39.5 | ||||
| Tumor size (mm) | <50 | 62 | 36.3 | 0.903 | ||
| ≥50 | 69 | 33.6 | ||||
| T category | T1–T3 | 109 | 32.4 | 0.146 | ||
| T4 | 22 | 47.5 | ||||
| Histopathological grading | G1/G2 | 113 | 31.2 | 0.006 | 1.00 | |
| G3 | 18 | 58.3 | 1.43 (0.98–2.07) | 0.063 | ||
| Lymphatic invasion | Absence | 58 | 35.4 | 0.745 | ||
| Presence | 73 | 34.8 | ||||
| Venous invasion | Absence | 66 | 30.5 | 0.220 | ||
| Presence | 65 | 39.8 | ||||
| N category | N1 | 92 | 29.6 | 0.034 | 1.00 | |
| N2 | 39 | 47.5 | 1.40 (0.74–2.65) | 0.302 | ||
| PDC | G1/G2 | 79 | 26.7 | 0.010 | 1.00 | |
| G3 | 52 | 47.5 | 1.85 (1.01–3.38) | 0.048 | ||
| Adjuvant chemotherapy | Absence | 59 | 39.8 | 0.282 | ||
| Presence | 72 | 31.3 | ||||
CRR, cumulative recurrence rate; HR, hazard ratio; CI, confidence interval; ASA-PS, American Society of Anesthesiologists physical status; PDC, poorly differentiated cluster.
Table 3.
Univariate and multivariate analyses of prognostic factors for relapse-free survival (RFS)
| Variable | Modality | n | Univariate | Multivariate | ||
|---|---|---|---|---|---|---|
| 5-y RFS (%) | P value | HR (95% CI) | P value | |||
| Age (years) | <65 | 51 | 62.1 | 0.298 | ||
| ≥65 | 80 | 54.1 | ||||
| Sex | Male | 78 | 55.4 | 0.633 | ||
| Female | 53 | 59.3 | ||||
| ASA-PS | 1/2 | 114 | 58.6 | 0.154 | ||
| 3/4 | 17 | 47.1 | ||||
| Tumor location | Colon | 49 | 68.3 | 0.018 | 1.00 | |
| Rectum | 82 | 50.4 | 1.78 (0.99–3.16) | 0.051 | ||
| Tumor size (mm) | <50 | 62 | 55.5 | 0.698 | ||
| ≥50 | 69 | 58.4 | ||||
| T category | T1–T3 | 109 | 58.5 | 0.445 | ||
| T4 | 22 | 50.0 | ||||
| Histopathological grading | G1/G2 | 113 | 61.0 | 0.011 | 1.00 | |
| G3 | 18 | 32.4 | 1.37 (0.99–1.89) | 0.058 | ||
| Lymphatic invasion | Absence | 58 | 58.6 | 0.624 | ||
| Presence | 73 | 55.7 | ||||
| Venous invasion | Absence | 66 | 62.4 | 0.258 | ||
| Presence | 65 | 51.3 | ||||
| N category | N1 | 92 | 61.1 | 0.139 | ||
| N2 | 39 | 47.1 | ||||
| PDC | G1/G2 | 79 | 66.0 | 0.004 | 1.00 | |
| G3 | 52 | 43.9 | 2.00 (1.20–3.33) | 0.008 | ||
| Adjuvant chemotherapy | Absence | 59 | 44.9 | 0.001 | 1.00 | |
| Presence | 72 | 67.1 | 2.20 (1.31–3.71) | 0.003 | ||
RFS, relapse-free survival; HR, hazard ratio; CI, confidence interval; ASA-PS, American Society of Anesthesiologists physical status; PDC, poorly differentiated cluster.
First sites of recurrence and PDC grade
The first recurrence was detected in the lung in 16 patients, liver in 13, local site in 9, extraregional lymph node in 9, peritoneum in 4, adrenal gland in 1 and brain in 1. In nine patients, more than two organs were involved. PDC G3 was significantly associated with cumulative extraregional lymph node metastasis (P = 0.007), while PDC G1/G2 was not associated with first sites of recurrence.
Adjuvant chemotherapy status and other clinicopathological characteristics
Compared with the chemotherapy group, the surgery-alone group was significantly associated with Age ≥65 (P < 0.001) and ASA-PS 3,4 (P = 0.035), while there were no significant associations between adjuvant chemotherapy status and other clinicopathological characteristics (Table 4).
Table 4.
Association between adjuvant chemotherapy status and other clinicopathological characteristics
| Chemotherapy group | Surgery-alone group | P value | |
|---|---|---|---|
| (n = 72) | (n = 59) | ||
| Age | |||
| <65 | 39 | 12 | <0.001 |
| ≥65 | 33 | 47 | |
| Sex | |||
| Male | 39 | 39 | 0.211 |
| Female | 33 | 20 | |
| ASA-PS | |||
| 1, 2 | 67 | 47 | 0.035 |
| 3, 4 | 5 | 12 | |
| Tumor location | |||
| Colon | 29 | 20 | 0.474 |
| Rectum | 43 | 39 | |
| Tumor size (mm) | |||
| <50 | 36 | 26 | 0.598 |
| ≥50 | 36 | 33 | |
| Tumor stage | |||
| T1–T3 | 59 | 50 | 0.815 |
| T4 | 13 | 9 | |
| Histopathological grading | |||
| G1, G2 | 62 | 51 | 1.000 |
| G3 | 10 | 8 | |
| Lymphatic invasion | |||
| Absence | 30 | 28 | 0.596 |
| Presence | 42 | 31 | |
| Venous invasion | |||
| Absence | 32 | 34 | 0.161 |
| Presence | 40 | 25 | |
| Nodal involvement | |||
| N1 | 48 | 44 | 0.344 |
| N2 | 24 | 15 | |
| PDC | |||
| G1, G2 | 41 | 38 | 0.473 |
| G3 | 31 | 21 | |
PDC, poorly differentiated cluster; ASA-PS, American Society of Anesthesiologists physical status.
CRR according to 5-FU-based adjuvant chemotherapy in PDC G1/G2 and PDC G3 patients
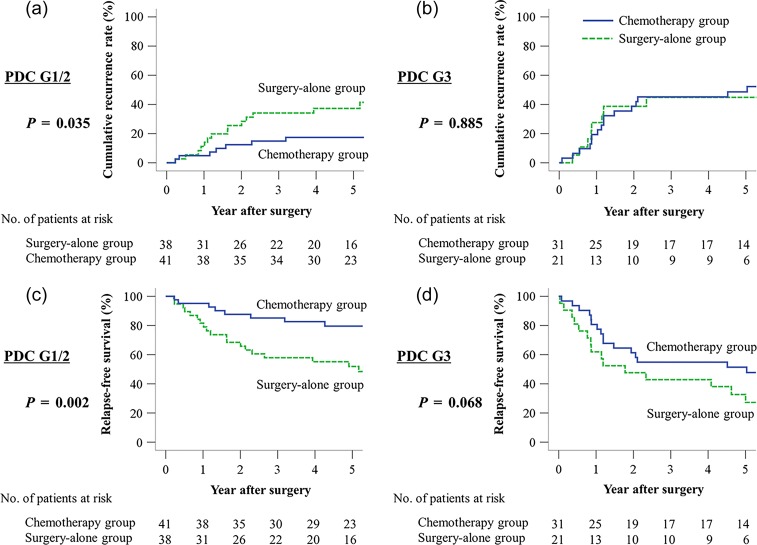
Among PDC G1/G2 patients, a significant difference in the 5-year CRR was observed between the chemotherapy group and surgery-alone group (17.4% vs. 37.3%, P = 0.035) (Fig. 4a). Conversely, among PDC G3 patients, no significant difference in 5-year CRR was observed between the chemotherapy group and surgery-alone group (48.6% vs. 44.8%, P = 0.885) (Fig. 4b).
Figure 4.
Comparative CRR and RFS curves of patients with or without 5-fluorouracil (5-FU)-based adjuvant chemotherapy in stage III CRC. (a) CRR of PDC G1/G2 patients, (b) CRR of PDC G3 patients, (c) RFS of PDC G1/G2 patients, (d) RFS of PDC G3 patients.
RFS according to 5-FU-based adjuvant chemotherapy in PDC G1/G2 and PDC G3 patients
Among PDC G1/G2 patients, a significant difference in the 5-year RFS was observed between the chemotherapy group and surgery-alone group (79.5% vs. 51.9%, P = 0.002) (Fig. 4c). Conversely, among PDC G3 patients, no significant difference in 5-year RFS was observed between the chemotherapy group and surgery-alone group (51.4% vs. 32.7%, P = 0.068) (Fig. 4d).
Discussion
The importance of the PDC was first highlighted by Ueno et al. in 2008 (26). Recently, several studies showed that PDC predicted prognosis in CRC more accurately than other histopathological parameters such as histopathological grading, venous or lymphatic invasion, tumor depth and nodal status (17,18). Furthermore, the PDC grading system is associated with a more ‘proportionate’ distribution of CRC tumors in each PDC grade (18). In our study, 39 (29.8%), 40 (30.5%) and 52 (39.7%) tumors were classified as PDC G1, G2 and G3, respectively. On the other hand, histopathological grading is associated with a more ‘disproportionate’ distribution of CRC tumors (18). In our study, 20 (15.3%), 93 (71.0%) and 18 (13.7%) tumors were classified as G1, G2 and G3. Therefore, we suggest that PDC grading identifies high-risk patients for recurrence and it stands for malignant biology.
To date, 5-FU-based adjuvant chemotherapy is universally recommended for patients with stage III CRC (27,28). However, Ueno et al. reported that the PDC enables the selection of a group of advanced CRC patients with very favorable survival outcome, thereby preventing unnecessary post-operative adjuvant chemotherapy and intensive surveillance in these patients (26). Few valuable predictors of efficacy of adjuvant chemotherapy have been investigated in CRC patients. The NCCN guidelines state that there is no evidence of predictive value of any of the available multigene assays in terms of the potential benefit of adjuvant chemotherapy (27). In this study, we demonstrated that PDC G1/G2 may be a useful predictor of response to 5-FU-based adjuvant chemotherapy in stage III CRC patients. Conversely, we speculate that CRC with PDC G3 may be resistant to 5-FU-based adjuvant chemotherapy.
Nowadays, the oxaliplatin-based regimen is the standard adjuvant chemotherapy regimen for stage III CRC (7,29). However, oxaliplatin is associated with significant side effects such as peripheral neuropathy or allergic reactions (7,29). Among the PDC G3 patients in this study, no significant differences in 5-year CRR and RFS were observed between the chemotherapy group and surgery-alone group (P = 0.885 and P = 0.068, respectively). These results indicate that the 5-FU-based regimen may not improve the prognosis of stage III CRC patients with PDC G3. Among those with PDC G1/G2, on the other hand, significant differences in 5-year CRR and RFS were observed between the 5-FU-based chemotherapy and the surgery-alone groups (P = 0.035 and P = 0.002, respectively). Therefore, the removal of oxaliplatin from adjuvant chemotherapy may be possible in stage III CRC patients with PDC G1/G2 to avoid these side effects. Thus, we consider that the PDC grade may play an important role in the selection of adjuvant chemotherapy regimen for stage III CRC.
In this study, we showed no significant differences in both 5-year CRR and RFS of the PDC G3 patients between the chemotherapy group and surgery-alone group. However, RFS of the PDC G3 patients in the surgery-alone group tended to be worse (P = 0.068). We speculate that the death caused by other diseases may make the RFS of the surgery-alone group worse. In Table 4, we demonstrated the differences of clinicopathological characteristics between the two groups: compared with the chemotherapy group, the surgery-alone group was significantly associated with Age ≥65 (P < 0.001) and ASA-PS 3,4 (P = 0.035).
Based on several recent studies, we think that PDC is associated with drug resistance, in which epithelial mesenchymal transition (EMT) is involved. Both PDC and cancer cells undergoing EMT have lost expression of E-cadherin and are closely associated with an upregulated Wnt/β-catenin signaling pathway (30–33). In their study of CRC progression, Brabletz et al. found that tumor cells at the tumor–host interface expressed EMT-associated and stemness-associated genes, which suggests a relationship between EMT and cancer stem cells (CSCs) in CRC (34). Despite limited understanding of the mechanisms causing CSC-related drug resistance, one of the hypotheses is that drug resistance is caused by overexpression of drug transporters and DNA repair enzymes in CSCs (35–37). Further studies such as immunohistochemical assays to identify markers of EMT or CSC in PDC are needed to elucidate the mechanism of resistance to 5-FU-based adjuvant chemotherapy in CRC patients with PDC G3.
This study has some potential limitations. This was a non-randomized, retrospective study performed at one institution. This study included the small sample size, and the selection bias due to limited inclusion criteria. A variety of 5-FU-based chemotherapy regimens such as infusional 5-FU/LV, UFT/LV, capecitabine or S-1 were administered in the chemotherapy group. Furthermore, ‘oxaliplatin-based’ regimens were excluded. A randomized controlled study comparing the oxaliplatin-based regimen with the 5-FU-based regimen is needed to clarify the efficacy of oxaliplatin as adjuvant chemotherapy in stage III CRC with PDC G3.
In conclusion, the efficacy of 5-FU-based adjuvant chemotherapy in stage III CRC differs according to PDC grade. The presence of PDC G1/G2 predicts a significant benefit from 5-FU-based adjuvant chemotherapy, whereas the presence of PDC G3 predicts a poor response to this regimen.
Authors’ contributions
Dr Tajima and Dr Shimada contributed to the conception and design of the study. Dr Tajima and Dr Shimada contributed to analysis and interpretation of data. Dr Tajima, Dr Shimada, Dr Kameyama, Dr Yagi, Dr Okamura and Dr Kobayashi contributed to collection and assembly of data. Dr Tajima contributed to drafting of the article. Dr Tajima, Dr Shimada and Dr Kosugi contributed to critical revision of the article for important intellectual content. Dr Wakai gave final approval for submission of the article.
Funding
This study was supported in part by a Grant-in-Aid for Scientific Research (No. 15K10130 to Y.S.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Conflict of interest statement
The authors declare no conflict of interest.
References
- 1. DeSantis CE, Lin CC, Mariotto AB, et al. . Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin 2014;64:252–71. [DOI] [PubMed] [Google Scholar]
- 2. Sargent D, Sobrero A, Grothey A, et al. . Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 2009;27:872–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gill S, Loprinzi CL, Sargent DJ, et al. . Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much. J Clin Oncol 2004;22:1797–806. [DOI] [PubMed] [Google Scholar]
- 4. Twelves C, Wong A, Nowacki MP, et al. . Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696–704. [DOI] [PubMed] [Google Scholar]
- 5. Moertel CG, Fleming TR, Macdonald JS, et al. . Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med 1990;322:352–8. [DOI] [PubMed] [Google Scholar]
- 6. Saini A, Norman AR, Cunningham D, et al. . Twelve weeks of protracted venous infusion of fluorouracil (5-FU) is as effective as 6 months of bolus 5-FU and folinic acid as adjuvant treatment in colorectal cancer. Br J Cancer 2003;88:1859–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. André T, Boni C, Mounedji-Boudiaf L, et al. . Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343–51. [DOI] [PubMed] [Google Scholar]
- 8. Yothers G, O'Connell MJ, Allegra CJ, et al. . Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011;29:3768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haller DG, Tabernero J, Maroun J, et al. . Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465–71. [DOI] [PubMed] [Google Scholar]
- 10. Fisher ER, Sass R, Palekar A, Fisher B, Wolmark N. Dukes’ classification revisited. Findings from the National Surgical Adjuvant Breast and Bowel Projects (Protocol R-01). Cancer 1989;64:2354–60. [DOI] [PubMed] [Google Scholar]
- 11. Freedman LS, Macaskill P, Smith AN. Multivariate analysis of prognostic factors for operable rectal cancer. Lancet 1984;2:733–6. [DOI] [PubMed] [Google Scholar]
- 12. Greene FL, Stewart AK, Norton HJ. A new TNM staging strategy for node-positive (stage III) colon cancer: an analysis of 50,042 patients. Ann Surg 2002;236:416–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamilton SR, Bosman FT, Boffetta P, et al. . Carcinoma of the colon and rectum In: Bosman FT, Carneiro F, Hruban RH, et al. editors. WHO Classification of Tumours of the Digestive System. Lyon: International Agency for Research on Cancer (IARC), 2010;134–146. [Google Scholar]
- 14. Blenkinsopp WK, Stewart-Brown S, Blesovsky L, Kearney G, Fielding LP. Histopathology reporting in large bowel cancer. J Clin Pathol 1981;34:509–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deans GT, Heatley M, Anderson N, et al. . Jass’ classification revisited. J Am Coll Surg 1994;179:11–7. [PubMed] [Google Scholar]
- 16. Thomas GD, Dixon MF, Smeeton NC, Williams NS. Observer variation in the histological grading of rectal carcinoma. J Clin Pathol 1983;36:385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ueno H, Hase K, Hashiguchi Y, et al. . Site-specific tumor grading system in colorectal cancer: multicenter pathologic review of the value of quantifying poorly differentiated clusters. Am J Surg Pathol 2014;38:197–204. [DOI] [PubMed] [Google Scholar]
- 18. Ueno H, Kajiwara Y, Shimazaki H, et al. . New criteria for histologic grading of colorectal cancer. Am J Surg Pathol 2012;36:193–201. [DOI] [PubMed] [Google Scholar]
- 19. Edge SB, Byrd DR, Compton CC, et al. . AJCC Cancer Staging Manual. 7th edn New York, NY: Springer, 2010. [Google Scholar]
- 20. Petrelli N, Douglass HO Jr, Herrera L, et al. . The modulation of fluorouracil with leucovorin in metastatic colorectal carcinoma: a prospective randomized phase III trial. Gastrointestinal Tumor Study Group. J Clin Oncol 1989;7:1419–26. [DOI] [PubMed] [Google Scholar]
- 21. Douillard JY, Hoff PM, Skillings JR, et al. . Multicenter phase III study of uracil/tegafur and oral leucovorin versus fluorouracil and leucovorin in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2002;20:3605–16. [DOI] [PubMed] [Google Scholar]
- 22. Carmichael J, Popiela T, Radstone D, et al. . Randomized comparative study of tegafur/uracil and oral leucovorin versus parenteral fluorouracil and leucovorin in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2002;20:3617–27. [DOI] [PubMed] [Google Scholar]
- 23. Yoshida M, Ishiguro M, Ikejiri K, et al. . S-1 as adjuvant chemotherapy for stage III colon cancer: a randomized phase III study (ACTS-CC trial). Ann Oncol 2014;25:1743–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Buyse M, Zeleniuch-Jacquotte A, Chalmers TC. Adjuvant therapy of colorectal cancer. Why we still don't know. JAMA 1988;259:3571–8. [PubMed] [Google Scholar]
- 25. Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer 2007;7:573–84. [DOI] [PubMed] [Google Scholar]
- 26. Ueno H, Mochizuki H, Hashiguchi Y, et al. . Histological grading of colorectal cancer: a simple and objective method. Ann Surg 2008;247:811–8. [DOI] [PubMed] [Google Scholar]
- 27. National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology, Colon Cancer. http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. Published November 2015 (13 November 2016, date last accessed).
- 28. National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology, Rectal Cancer. http://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Published April 2016 (13 November 2016, date last accessed).
- 29. André T, Boni C, Navarro M, et al. . Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109–16. [DOI] [PubMed] [Google Scholar]
- 30. Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev 2009;28:151–66. [DOI] [PubMed] [Google Scholar]
- 31. Tian X, Liu Z, Niu B, et al. . E-cadherin/β-catenin complex and the epithelial barrier. J Biomed Biotechnol 2011;2011:567305.doi:10.1155/2011/567305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kajiwara Y, Ueno H, Hashiguchi Y, et al. . Expression of l1 cell adhesion molecule and morphologic features at the invasive front of colorectal cancer. Am J Clin Pathol 2011;136:138–44. [DOI] [PubMed] [Google Scholar]
- 33. Barresi V, Branca G, Vitarelli E, Tuccari G. Micropapillary pattern and poorly differentiated clusters represent the same biological phenomenon in colorectal cancer: a proposal for a change in terminology. Am J Clin Pathol 2014;142:375–83. [DOI] [PubMed] [Google Scholar]
- 34. Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells – an integrated concept of malignant tumour progression. Nat Rev Cancer 2005;5:744–9. [DOI] [PubMed] [Google Scholar]
- 35. Styczynski J, Drewa T. Leukemic stem cells: from metabolic pathways and signaling to a new concept of drug resistance targeting. Acta Biochim Pol 2007;54:717–26. [PubMed] [Google Scholar]
- 36. Zhou S, Schuetz JD, Bunting KD, et al. . The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med 2001;7:1028–34. [DOI] [PubMed] [Google Scholar]
- 37. Patrawala L, Calhoun T, Schneider-Broussard R, Zhou J, Claypool K, Tang DG. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2- cancer cells are similarly tumorigenic. Cancer Res 2005;65:6207–19. [DOI] [PubMed] [Google Scholar]