Figure 1.
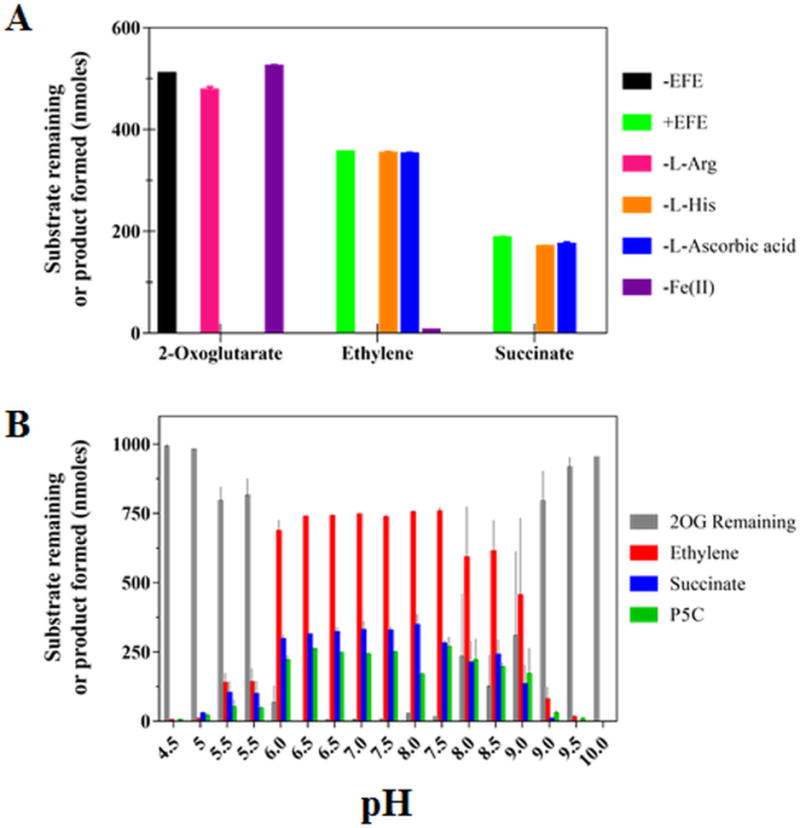
Enzymatic activity of EFE. (A) Components essential for the EFE reaction. EFE (254 nM, except for the EFE-free control sample) was incubated in 1 mL of 40 mM HEPES buffer, pH 7.5, containing (except where the component was eliminated) 0.5 mM 2OG, 0.5 mM L-Arg, 10 mM L-His, 0.2 mM Fe(II), and 1 mM L-ascorbic acid, followed by analysis of the remaining 2OG and production of ethylene and succinate. (B) pH dependence of the EFE reaction. A 2 mL reaction in 40 mM buffer (sodium acetate, pH 4.5–5.5, MES, pH 5.5–6.5, HEPES, pH 6.5–8.0, bis-Tris propane, pH 7.5–9.0, and CHES, pH 9.0–10) contained EFE (505 nM), 0.5 mM 2OG, 0.5 mM L-Arg, 0.2 mM (NH4)2Fe(SO4)2, and 0.4 mM L-ascorbic acid. The reactions were incubated at 25 °C for 80 min, terminated with HCl, and assessed for the remaining 2OG and production of ethylene, succinate, and P5C. Error bars the represent standard errors for n = 2.
