Figure 3.
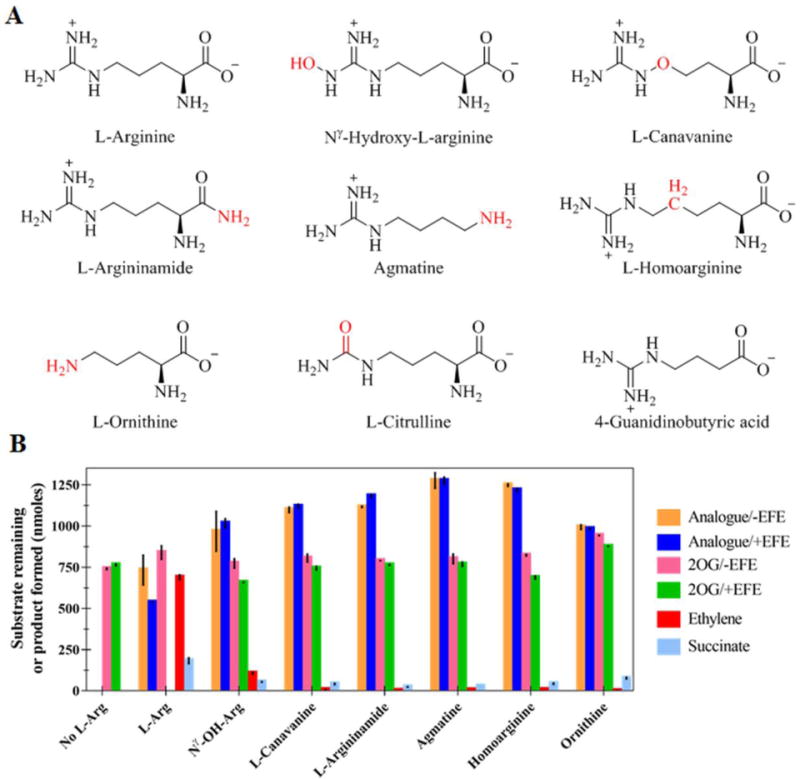
Reactions of EFE in the presence of various L-Arg analogues. (A) Structures of the L-Arg derivatives with differences from L-Arg highlighted in red. (B) Concentrations of 2OG remaining or ethylene and succinate produced after the reaction (or the no-enzyme controls) for various L-Arg analogues and a no-analogue control. Solutions (2 mL) containing 0.5 mM 2OG, 0.5 mM L-Arg analogue, 0.2 mM Fe(II), 0.4 mM L-ascorbic acid, and (when present) 252 nM EFE in 10 mM NH4HCO3 buffer, pH 7.5 were incubated at 25 °C for 80 min and terminated with formic acid. Error bars represent standard errors for n = 2.
