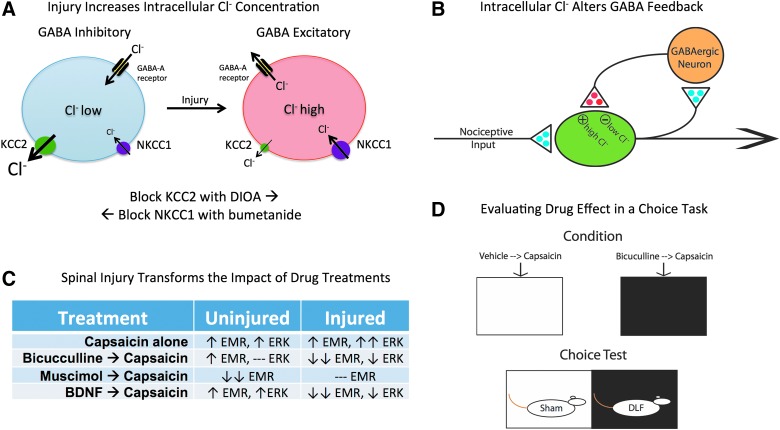
FIG. 3.
GABA function is transformed by spinal injury. (A) In adult uninjured animals (left), the combined action of the cotransporters KCC2 and NKCC1 maintain a low intracellular concentration of Cl−. Engaging the GABA-A receptor allows Cl− to flow into the cell, causing the hyperpolarization that underlies its inhibitory effect. Spinal injury (right) lowers membrane-bound KCC2, which reduces the outward flow of Cl− and increases intracellular Cl− concentrations. Under these conditions, engaging the GABA-A receptor can allow Cl− to exit the cell, which would have a depolarizing effect. Intracellular Cl− concentrations can be experimentally manipulated in adult animals using drugs that disrupt KCC2 (DIOA) and NKCC1 (bumetanide) function. In intact animals, DIOA retards the outward flow of Cl− through the KCC2 channel, which increases intracellular Cl− concentrations (bottom). In spinally transected animals, bumetanide lowers the flow of Cl− into the cell, reducing the intracellular Cl− concentration. (B) In uninjured animals, GABAergic neurons within the spinal cord are engaged by afferent nociceptive input and may provide a form of negative feedback, which would help prevent overexcitation. After spinal injury, the reduction in membrane-bound KCC2 would transform how GABA release affects nociceptive circuits, causing it to have a depolarizing [excitatory (+)] effect that could contribute to the development of nociceptive sensitization. Excitatory (glutamatergic) transmitters are indicated in blue and inhibitory (GABAergic) transmitters are colored red. (C) Summary of how alternative treatments affect capsaicin-induced EMR and ERK activation in uninjured and spinally injured rats. Stronger effects are indicated with a double arrow. A dashed line indicates that a drug treatment has no effect. (D) The task used to examine how alterations in spinal GABA function affect pain signals relayed to the brain. Behavioral data indicated that lesioning the DLF transformed how i.t. bicuculline affects capsaicin-induced EMR, causing it to have an anti-allodynic-like effect. If this modification also alters pain signaling to the brain, i.t. bicuculline should attenuate capsaicin-induced pain and reduce its capacity to support a conditioned aversion. To evaluate this possibility, DLF-lesioned and sham-operated rats were given bicuculline or its vehicle i.t. before capsaicin treatment and being placed in one of two distinctive contexts. Each subject received both types of conditioning trials, which were spaced across days. Both treatment order and which environment was paired with bicuculline were counterbalanced across subjects. Rats were then given a choice between the two conditioning chambers. As predicted, DLF-lesioned rats preferred the environment where they had experienced i.t. bicuculline before capsaicin treatment. If anything, sham-operated rats exhibited the opposite preference. BDNF, brain-derived neurotrophic factor; DIOA, dihydro-indenyl-oxy alkanoic acid; DLF, dorsolateral funiculus; EMR, enhanced mechanical reactivity; ERK, extracellular signal-regulated kinases; GABA, gamma-aminobutyric acid; GABA-A, GABA type A; i.t., intrathecal; KCC2, K+-Cl− cotransporter 2; NKCC1, Na+-K+- Cl− cotransporter 1.