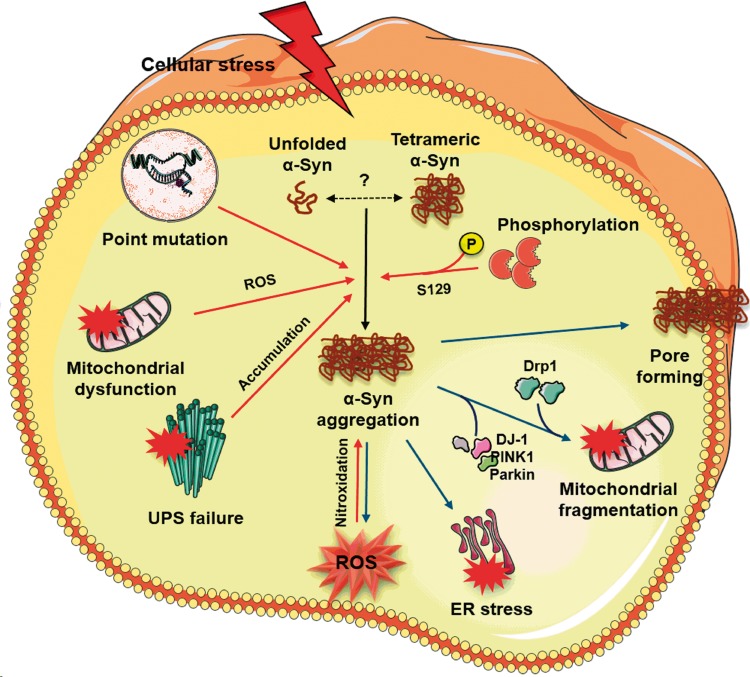
Figure 2.
Mechanism of oligomeric α-Syn-mediated cellular toxicity in PD. In a normal physiological condition, α-Syn has long been known to exist as an unfolded monomer. However, recent evidence suggests that α-Syn under normal physiological state may exist as tetramers that resist aggregation. Tetrameric α-Syn species become destabilized and prone to aggregation due to several pathologic factors including point mutations, ROS generated by dysfunctional mitochondria and post-translational modifications like phosphorylation and nitroxidation. Concomitantly, impaired ubiquitin-proteasomal system (UPS) may not effectively clear the misfolded proteins resulting in the accumulation of oligomeric α-Syn species. α-Syn aggregates, in turn, increase cellular toxicity by several mechanisms. The α-Syn oligomers (1) permeabilize the lipid bilayer by forming Ca2+-permeable pore-like complexes thereby disturbing intracellular calcium homeostasis, (2) promote mitochondrial fragmentation by directly interacting with mitochondrial complex I, recruiting mitochondrial fission protein Drp1 and inhibiting DJ-1/PINK1/Parkin proteins that are known to maintain mitochondrial integrity, (3) activate the prolonged unfolded protein response (UPR) by accumulating within the ER lumen, and (4) generate mitochondria-independent ROS via redox activities with metal ions.