Abstract
Plant defenses inducible by herbivorous arthropods can determine performance of subsequent feeding herbivores. We investigated how infestation of tomato (Solanum lycopersicum) plants with the Western flower thrips (Frankliniella occidentalis) alters host plant suitability and foraging decisions of their conspecifics. We explored the role of delayed-induced jasmonic acid (JA)-mediated plant defense responses in thrips preference by using the tomato mutant def-1, impaired in JA biosynthesis. In particular, we investigated the effect of thrips infestation on trichome-associated tomato defenses. The results showed that when offered a choice, thrips preferred non-infested plants over infested wild-type plants, while no differences were observed in def-1. Exogenous application of methyl jasmonate restored the repellency effect in def-1. Gene expression analysis showed induction of the JA defense signaling pathway in wild-type plants, while activating the ethylene signaling pathway in both genotypes. Activation of JA defenses led to increases in type-VI leaf glandular trichome densities in the wild type, augmenting the production of trichome-associated volatiles, i.e. terpenes. Our study revealed that plant-mediated intraspecific interactions between thrips are determined by JA-mediated defenses in tomato. We report that insects can alter not only trichome densities but also the allelochemicals produced therein, and that this response might depend on the magnitude and/or type of the induction.
Keywords: Frankliniella occidentalis, Jasmonic acid, Thrips, Tomato, Trichomes, Volatiles
Introduction
Herbivore feeding can cause extensive damage to plants and induce dramatic physiological, biochemical and physical changes. Many of these changes are associated with defense against herbivores and are characterized by the production of defensive compounds and morphological structures that hinder the attackers (Karban and Myers 1989, Kessler and Baldwin 2001, Howe and Jander 2008). Induction of these defenses can occur within a few hours or days, i.e. rapid induced response, affecting development of early colonizing herbivores, or within weeks or months, i.e. delayed induced response, thus altering the establishment of herbivores that subsequently feed on wounded plants (Karban and Baldwin 1997, Denno and Kaplan 2007). Plant-induced defenses can, therefore, play a central role in modulating intra- or interspecific interactions among herbivores (Dalin and Björkman 2003, Poelman et al. 2008, Erb et al. 2011). These plant defense responses are mainly regulated by three phytohormones: jasmonic acid (JA), salicylic acid (SA) and ethylene (ET). JA and ET are generally associated with plant defense responses against necrotrophic pathogens and herbivorous arthropods. In particular, activation of the JA signaling pathway is characterized by the induction of defensive compounds in vegetative tissues such as secondary metabolites (e.g. polyamines, quinones, terpenoids, alkaloids, phenylpropanoids, glucosinolates and antioxidants) (van der Fits and Memelink 2000, Keinänen et al. 2001, Chen et al. 2006), proteins (e.g. polyphenol oxidases and proteinase inhibitors) (Farmer and Ryan 1990, Thaler et al. 1996) and leaf trichomes (Boughton et al. 2005). While some of these plant responses are displayed within 30 min to 24 h after herbivore attack (Fowler et al. 2009, Wu and Baldwin 2009), trichome induction in newly formed leaves constitutes a more delayed defense response, i.e. detected several weeks after the initial induction (Boughton et al. 2005, Peiffer et al. 2009). Plant trichomes are hairy leaf structures developed from epidermal cells that can be classified as non-glandular or glandular. The latter are responsible for the production of plant secondary metabolites that are stored and/or secreted onto the leaf surface (Glas et al. 2012). Induction of trichome densities after herbivore attack has been described for different plant species such as willox (Salix cinerea), black mustard (Brassica nigra) and tomato (Solanum lycopersicum) (Dalin et al. 2008, Tian et al. 2012a), among others, and has been proved to be herbivore species specific (Traw and Dawson 2002). Though numerous studies have documented the herbivore-mediated induction of plant trichome densities, to the best of our knowledge no effect on the allelochemicals produced in the glandular-type trichomes has been described so far.
Western flower thrips Frankliniella occidentalis [Pergande] is a plant cell content feeder that severely affects vegetable and ornamental production worldwide (Reitz 2009). Thrips feeding can induce JA signaling in plants, and this response is required for mounting the effective plant defenses against this insect in Arabidopsis (De Vos et al. 2005, Abe et al. 2008, Abe et al. 2009) and tomato (Li et al. 2002, Kawazu et al. 2012). Moreover, artificial induction of JA-mediated defenses was reported to increase resistance to thrips in cotton (Aphis gossypii) (Omer et al. 2001) and Chinese cabbage (Brassica rapa) (Abe et al. 2009). To date, characterization of plant defense responses to thrips has focused on early events, i.e. 0–96 h, after infestation. Among these events, thrips feeding has been reported to induce the release of an array of volatile organic compounds (VOCs) in Nicotiana tabacum plants (Delphia et al. 2007). Induced VOCs play an important role in plant defense. They are mediators of indirect defenses forming part of the plant’s arsenal to repel herbivores, increase plant toxicity (Kessler and Baldwin 2001, De Moraes et al. 2001) or attract herbivore natural enemies (Dicke and van Loon 2000, Robert et al. 2012). In this sense, Agrawal and Colfer (2000) described that thrips-infested cotton plants were less preferred by subsequent colonizing conspecifics. Odor cues emanating from infested plants were suggested to affect thrips choice, but no further studies on the mechanisms operating in these plant–thrips interactions have been described. Some studies have demonstrated that activation of plant defenses by other arthropod herbivores can affect thrips preference and survival (Delphia et al. 2007), highlighting the central role of induced defenses in shaping the community of herbivores (Poelman et al. 2008, Erb et al. 2011, Glas et al. 2014).
In the present study, we investigated whether JA-associated defense responses induced by thrips affected host plant acceptance by its conspecifics in tomato (S. lycopersicum) using the tomato mutant def-1, impaired in JA-induced defense responses (Lightner et al. 1993, Howe et al. 1996). Among the induced defenses, we analyzed changes in the gene expression of JA-, SA- and ET-responsive markers, type-VI leaf glandular trichome densities and their main associated volatile allelochemicals, terpenes (Wagner 1991, Besser et al. 2009, McDowell et al. 2011). Type-VI glandular trichomes are controlled by the JA pathway (Li et al. 2004, Boughton et al. 2005, Yoshiba et al. 2009, Maes and Goossens 2010) and can act as physical barriers, but also as chemical factories for production of toxic and repellent substances against arthropod herbivores and pathogens (Kang et al. 2010a, Kang et al. 2010b). This trichome type constitutes the main glandular trichome in Castlemart and def-1 tomato leaves (Peiffer et al. 2009). Alterations in type-VI glandular trichome density and associated allelochemicals might, therefore, influence tomato–thrips interactions. To determine whether thrips-mediated induced responses were similar to those activated by artificially induced JA signaling, we compared these plant defense responses with those triggered by the exogenous application of the JA derivate phytohormone methyl jasmonate (MeJA). In addition, we further addressed whether type-VI trichome induction and production of their associated volatiles were positively correlated to the silver damage symptoms caused by thrips feeding.
Results
Induced JA defenses play a key role in tomato-mediated intraspecific interactions for thrips
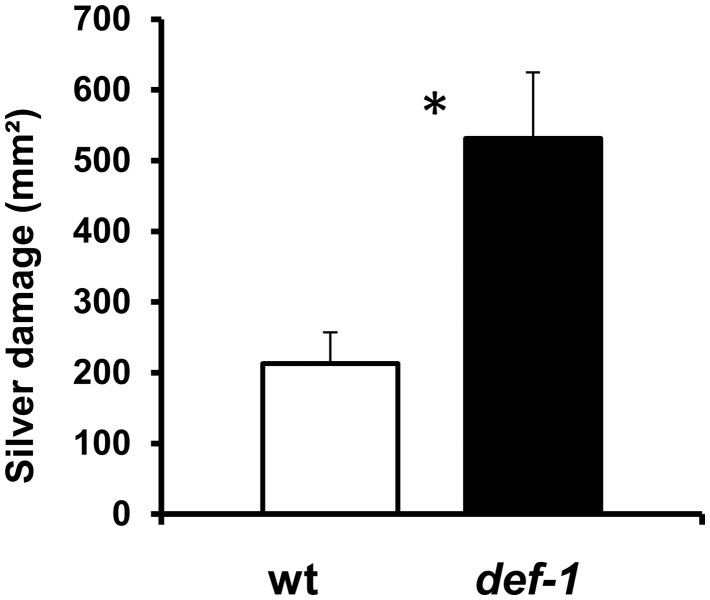
Thrips-infested def-1 plants showed significantly higher silver damage symptoms than wild-type plants (Student’s t-test, t = 2.77, P = 0.017) (Fig. 1). Similar results were observed in a replicated experiment (Supplementary Fig. S1).
Fig. 1.
Effect of JA-mediated plant defense responses on tomato resistance to Frankliniella occidentalis. Mean (± SEM, n = 6–7) plant damage caused by thrips infestation was measured in wild-type (wt) and def-1 plants 12 d after thrips release. Representative data from one of the replicated experiments are shown. Asterisks denote a significant difference between wt and def-1 plants compared by unpaired t-test at P ≤ 0.05.
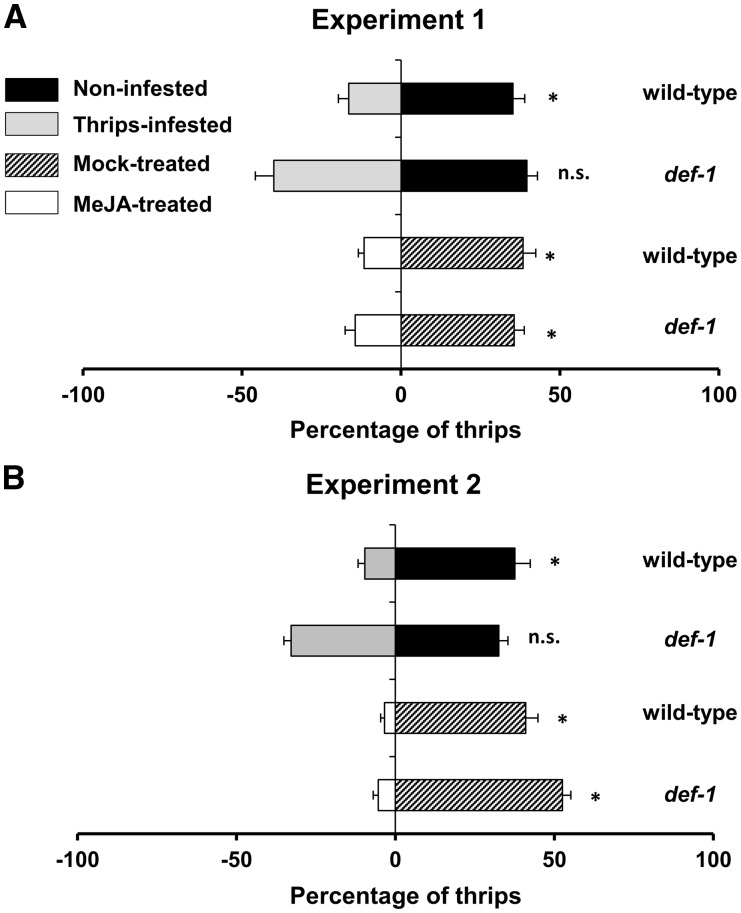
To determine whether induction of JA-associated defenses by thrips infestation or MeJA affects thrips preference in wild-type and def-1 plants, leaf disc dual-choice assays were performed in two replicated experiments (Fig. 2). Thrips showed higher preference for leaf discs taken from non-infested over infested wild-type plants (P ≤ 0.05) (Fig. 2A, B). No significant differences were observed between leaf discs taken from non-infested and infested def-1 plants. Exogenous MeJA application significantly increased the repellency against thrips in wild-type and def-1 plants (P ≤ 0.05).
Fig. 2.
Effect of a prior thrips infestation or exogenous application of MeJA in wild-type (wt) and def-1 plants on thrips preference, 12 d after the initial treatment, as tested in a dual-choice leaf disc assay. Percentage (± SEM, n = 25–35) of thrips settled on leaf discs taken from non-infested vs. thrips-infested wt plants, non-infested vs. thrips-infested def-1 plants, mock-treated vs. MeJA-treated wt plants and mock-treated vs. MeJA-treated def-1 plants in two replicated experiments (A, B). Averaged preference data recorded at 0.5, 1, 2, 3 and 4 h after thrips release are shown. Data were analyzed by Wilcoxon matched-pairs signed-rank test. Asterisks denote significant differences at P ≤ 0.05.
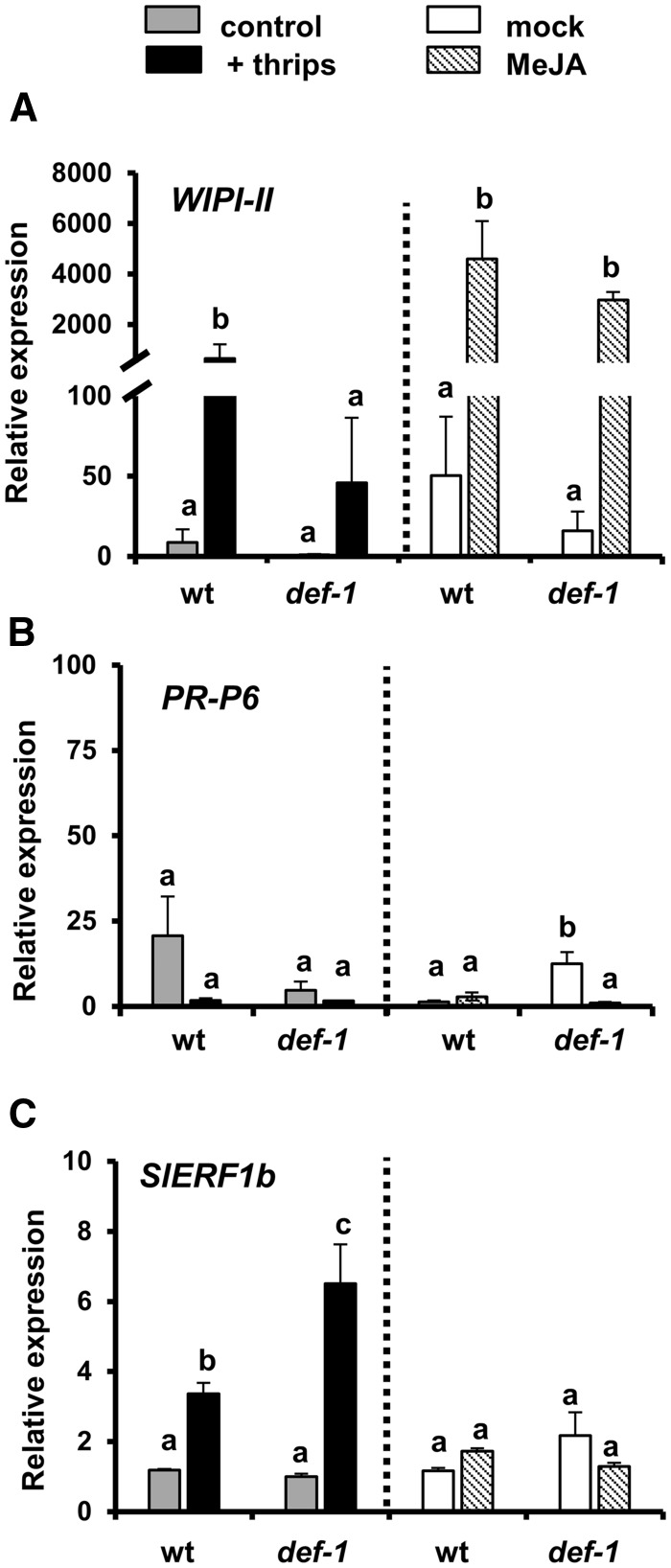
To test whether thrips infestation or MeJA treatment activate the JA, SA or ET signaling pathways, expression levels of the responsive gene markers WIPI-II (wound inducible proteinase inhibitor II), PR-P6 (pathogenesis-related protein 6) and SlERF1b (Solanum lycopersicum ethylene-responsive factor), respectively, were analyzed at 12 d after initial treatments (Fig. 3). Expression of WIPI-II was up-regulated by thrips infestation in wild-type plants, but not in def-1 [generalized linear model (GLM): Wald χ2 = 12.66, P < 0.001 for infestation treatment; Wald χ2 = 1.25, P = 0.262 for plant genotype; Wald χ2 = 5.22, P = 0.001 for the interaction] (Fig. 3A). Conversely, MeJA application induced the expression of WIPI-II in both wild-type and def-1 plants (GLM: Wald χ2 = 42.60, P < 0.001 for hormone treatment; Wald χ2 = 0.468, P = 0.494 for plant genotype; Wald χ2 = 0.99, P = 0.318 for the interaction). Expression levels of the SA marker PR-P6 did not differ in thrips-infested wild-type and def-1 plants when compared with their respective controls (GLM: Wald χ2 = 0.590, P = 0.443 for infestation treatment; Wald χ2 = 0.304, P = 0.581 for plant genotype; Wald χ2 = 2.82, P = 0.093 for the interaction) (Fig. 3B). Similarly, MeJA treatment did not affect expression levels of PR-P6 in wild-type and def-1 plants (GLM: Wald χ2 = 0.356, P = 0.06 for hormone treatment; Wald χ2 = 3.122, P = 0.077 for plant genotype; Wald χ2 = 0.591, P = 0.015 for the interaction). However, MeJA-treated def-1 plants showed lower levels of PR-P6 when compared with mock-treated plants, resulting in a statistically significant interaction between plant genotype and hormone treatment. A stronger and negative JA–SA cross-talk in the JA-defective mutant might explain the lower basal levels of this gene marker (Koornneef and Pieterse 2008). The ET response factor SlERF1b was induced after thrips infestation in def-1 and wild-type plants, this induction being significantly stronger in def-1 (GLM: Wald χ2 = 92.59, P < 0.001 for infestation treatment; Wald χ2 = 2.00, P = 0.157 for plant genotype; Wald χ2 = 7.33, P = 0.007 for the interaction) (Fig. 3C). Conversely, SlERF1b expression levels in wild-type and def-1 plants were not altered by MeJA (GLM: Wald χ2 = 0.118, P = 0.732 for hormone treatment; Wald χ2 = 3.66, P = 0.545 for plant genotype; Wald χ2 = 2.289, P = 0.130 for the interaction).
Fig. 3.
Relative transcript levels of the JA-responsive gene WIPI-II (A), the SA-responsive gene PRP-6 (B) and SlERF1b (C) were measured in non-infested, thrips-infested, mock-treated and MeJA-treated wild-type and def-1 plants at 12 d after initial treatment. Bars indicate mean ± SEM fold induction of each treatment group (n = 3 biological replicates, two technical replicates). Different letters above bars denote significant differences among groups compared by Fisher’s LSD test at P ≤ 0.05.
Activation of JA-associated defenses mediates induction of trichome densities upon thrips infestation in tomato
To investigate further the delayed induced JA-associated defenses that might determine host plant acceptance of thrips, changes in leaf type-VI glandular trichome-associated defenses were analyzed.
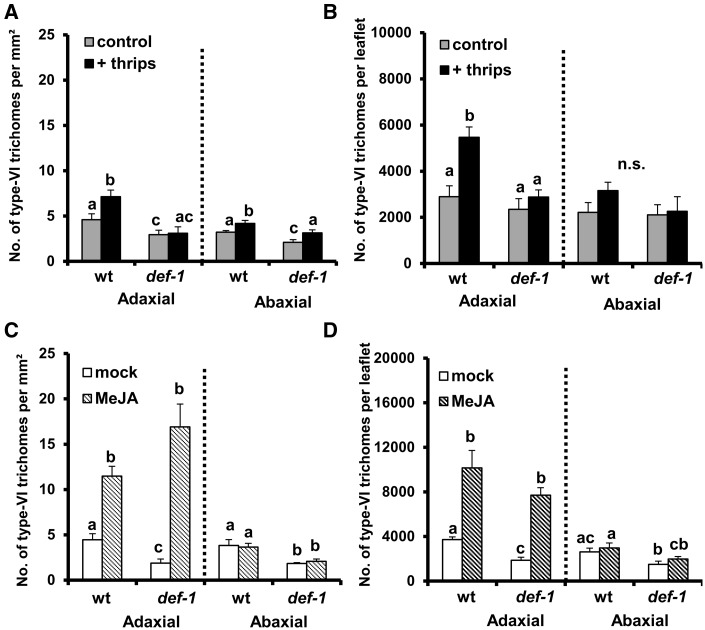
Wild-type plants infested with F. occidentalis showed increased type-VI glandular trichome densities on adaxial leaf sides of young leaves at 12 d after the initial treatment when compared with uninfested plants (GLM: Wald χ2 = 5.021, P = 0.025 for infestation treatment; Wald χ2 = 22.28, P < 0.001 for plant genotype; Wald χ2 = 3.97, P = 0.046 for the interaction) (Fig. 4A). No significant differences were observed between thrips-infested and non-infested def-1 plants, yet lower trichome densities were observed in non-infested def-1 plants compared with the wild-type. The total number of type-VI trichomes on adaxial leaf sides of thrips-infested wild-type plants was also higher than in non-infested plants (GLM: Wald χ2 = 15.99, P < 0.001 for infestation treatment; Wald χ2 = 16.32, P < 0.001 for plant genotype; Wald χ2 = 6.90, P = 0.009 for the interaction) (Fig. 4B). No differences were observed for def-1, but non-infested def-1 plants displayed lower trichome number compared with the wild type. Thrips infestation significantly increased type-VI trichome densities on abaxial leaf sides of wild-type and def-1 plants (GLM: Wald χ2 = 13.29, P < 0.001 for infestation treatment; Wald χ2 = 14.96, P < 0.001 for plant genotype; Wald χ2 = 0.000, P = 0.986 for the interaction) (Fig. 4A). However, when the total number of trichomes per leaflet was determined on abaxial leaf sides, no significant differences were observed between thrips-infested and non-infested wild-type or def-1 plants (GLM: Wald χ2 = 1.41, P = 0.235 for infestation treatment; Wald χ2 = 1.16, P = 0.280 for plant genotype; Wald χ2 = 0.713, P = 0.399 for the interaction) (Fig. 4B). MeJA treatment significantly increased type-VI trichome densities on adaxial leaf sides of wild-type and def-1 plants (GLM: Wald χ2 = 69.29, P < 0.001 for hormone treatment; Wald χ2 = 5.86, P = 0.016 for plant genotype; Wald χ2 = 8.30, P = 0.004 for the interaction) (Fig. 4C). Reduced type-VI trichome densities on adaxial leaf sides were observed in mock-treated def-1 plants when compared with the wild type. Although MeJA increased trichome densities in both plant genotypes, greater differences between mock-treated and MeJA-treated plants were observed for def-1, as shown by the significant interaction between plant genotype and hormone treatment. Similarly, the total number of trichomes on adaxial leaf sides was higher in MeJA-treated wild-type and def-1 plants (GLM: Wald χ2 = 114.10, P < 0.001 for hormone treatment; Wald χ2 = 17.7, P < 0.001 for plant genotype; Wald χ2 = 5.13, P < 0.001 for the interaction) (Fig 4D). As mock-treated def-1 plants displayed lower trichome number compared with the wild type, signficant differences in the magnitude of the induction were observed for this genotype. Type-VI trichome densities on abaxial leaf sides of wild-type and def-1 plants were not induced by MeJA, but mock-treated def-1 plants showed lower trichome densities than the wild type (GLM: Wald χ2 = 0.123, P = 0.726 for hormone treatment; Wald χ2 = 30.241, P < 0.001 for plant genotype; Wald χ2 = 0.219, P = 0.640 for the interaction) (Fig. 4C). Trichome number on abaxial leaf sides of wild-type and def-1 plants was not affected by MeJA treatment either, but mock-treated def-1 plants showed fewer trichomes when compared with the wild-type (GLM: Wald χ2 = 1.58, P = 0.208 for hormone treatment; Wald χ2 = 10.208, P < 0.001 for plant genotype; Wald χ2 = 0.039, P = 0.843 for the interaction) (Fig. 4D).
Fig. 4.
Type-VI glandular trichome density and total number per leaflet were evaluated in adaxial and abaxial leaf sides of leaflets taken from the third/fourth youngest leaf at 12 d after the initial treatments. Data show the mean (±SEM, n = 6–7) of type-VI leaf glandular trichome densities and total number of trichomes per leaflet in non-infested and thrips-infested wild-type (wt) and def-1 plants (A, B) and mock-treated and MeJA-treated wt and def-1 plants (C, D). Different letters above bars denote significant differences among groups within adaxial or abaxial data compared by Fisher’s LSD test at P ≤ 0.05.
To investigate further whether increases in trichome densities and number per leaflet were explained by changes in epidermal cell number, epidermal cell densities were determined. No significant differences in epidermal cell densities were observed between non-infested and thrips-infested wild-type and def-1 plants (GLM: Wald χ22 = 0.27, P = 0.602 for infestation treatment; Wald χ2 = 0.64, P = 0.424 for plant genotype; Wald χ2 = 0.604, P = 0.437 for the interaction) (Supplementary Fig. S2A). A significant reduction in epidermal cell densities was detected in MeJA-treated wild-type and def-1 plants when compared with their controls (GLM: Wald χ2 = 23, P < 0.001 for hormone treatment; Wald χ2 = 8.07, P = 0.004 for plant genotype; Wald χ2 = 2.5, P = 0.114 for the interaction) (Supplementary Fig. S2B). Hence, a larger size of epidermal cells was observed in young leaves of MeJA-treated wild-type and def-1 plants (Supplementary Fig. S2F, J). Although lower epidermal cell density was observed in mock-treated def-1 plants when compared with mock-treated wild-type plants, reduction of epidermal cell density by MeJA treatment was similar in both plant genotypes.
Thrips infestation alters leaf trichome-associated volatile production
Thirteen major compounds were detected in the leaf exudates of wild-type and def-1 tomato plants under the different treatments (Table 1; Supplementary Fig. S3). Among these, we identified the monoterpenes α-pinene, p-cymene, myrcene, δ-carene, α-phellandrene, α-terpinene, limonene, β-phellandrene, trans-β-ocimene, γ-terpinene, terpinolene and the sesquiterpene α-caryophyllene. These volatile compounds coincided with those commonly detected in tomato type-VI glandular trichomes (Kang et al. 2014).
Table 1.
Terpene content in leaf exudates of non-infested and thrips-infested wild-type and def-1 plants, and mock-treated and MeJA-treated wild-type and def-1 plants
| No. | Compound | Treatments | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Wild-type (µg g–1 FW) | def-1 (µg g–1 FW) | Wild-type (µg g–1 FW) | def-1 (µg g–1 FW) | ||||||
| Control | +Thrips | Control | +Thrips | Mock | MeJA | Mock | MeJA | ||
| 1 | α-Pinene | 2.57 ± 0.28 a | 3.32 ± 0.33 b | 1.53 ± 0.16 c | 1.90 ± 0.12 c | 2.95 ± 0.22 a | 9.97 ± 1.17 b | 1.25 ± 0.10 c | 13.13 ± 1.67 d |
| 2 | p-Cymene | 0.46 ± 0.21 a | 0.51 ± 0.15 ab | 0.15 ± 0.15 bc | 0.0 ± 0.0 c | 0.59 ± 0.24 a | 2.66 ± 0.28 b | 0.0 ± 0.0 c | 6.19 ± 1.06 b |
| 3 | Myrcene | 0.31 ± 0.13 a | 0.52 ± 0.09 a | 0.0 ± 0.0 b | 0.38 ± 0.12 a | 0.45 ± 15 a | 2.05 ± 0.24 ac | 0.096 ± 0.09 b | 2.9 ± 0.31 c |
| 4 | δ-Carene | 8.68 ± 0.96 a | 11.13 ± 1.10 b | 4.62 ± 0.72 c | 6.26 ± 0.66 ac | 9.71 ± 0.58 a | 57.83 ± 5.61 b | 3.77 ± 0.26 c | 58.50 ± 6.18 b |
| 5 | α-Phellandrene | 2.01 ± 0.24 a | 2.94 ± 0.22 b | 1.11 ± 0.14 c | 1.48 ± 0.14 ac | 2.48 ± 0.21 a | 13.10 ± 1.26 b | 0.87 ± 0.04 c | 14.73 ± 1.69 b |
| 6 | α-Terpinene | 0.57 ± 0.17 a | 1.27 ± 0.32 b | 0.087 ± 0.08 a | 0.24 ± 0.13 a | 0.63 ± 0.21 a | 4.40 ± 0.40 b | 0.08 ± 0.08 c | 4.85 ± 0.5 b |
| 7 | Limonene | 5.28 ± 0.63 a | 7.55 ± 0.81 b | 2.12 ± 0.73 c | 4.02 ± 0.37 ac | 6.94 ± 0.64 a | 24.68 ± 8.45 b | 2.38 ± 0.12 c | 39.44 ± 4.91 b |
| 8 | β-Phellandrene | 24.20 ± 6.22 a | 38.80 ± 4.57 b | 17.11 ± 3.66 a | 20.56 ± 2.29 a | 33.99 ± 2.22 a | 183.66 ± 14.51 b | 11.89 ± 0.71 c | 209.78 ± 26.45 b |
| 9 | trans-β-Ocimene | 0.73 ± 0.12 a | 0.79 ± 0.077 a | 0.0 ± 0.0 b | 0.10 ± 0.10 b | 0.71 ± 0.05 a | 2.25 ± 0.26 a | 0.0 ± 0.0 b | 2.22 ± 0.81 a |
| 10 | γ-Terpinene | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 a | 0.73 ± 0.0 b | 0.0 ± 0.0 a | 0.84 ± 0.24 b |
| 11 | Terpinolene | 0.0 ± 0.0 a | 0.06 ± 0.06 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0.94 ± 0.10 b | 0.0 ± 0.0 a | 1.10 ± 0.15 b |
| 12 | Unknown | 2.63 ± 0.27 a | 3.37 ± 0.22 b | 1.67 ± 0.14 c | 2.87 ± 0.44 ab | 0.408 ± 0.60 a | 5.82 ± 1.11 ac | 1.48 ± 0.33 b | 9.27 ± 1.98 c |
| 13 | β-Caryophyllene | 6.39 ± 0.72 a | 5.90 ± 0.88 ab | 3.11 ± 0.38 b | 4.32 ± 1.12 ab | 7.55 ± 1.14 a | 2.17 ± 0.16 a | 2.57 ± 0.98 a | 2.56 ± 1.14 a |
The mean and SEM (n = 4–7) of each volatile identified is shown.
Different letters denote significant differences among groups compared by LSD test at P ≤ 0.05.
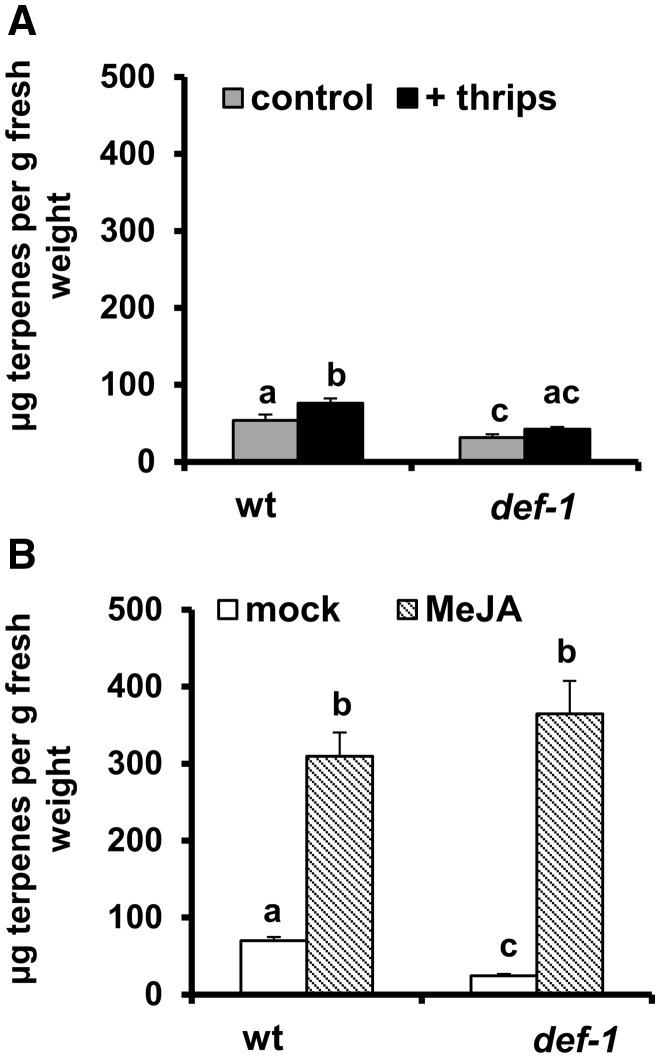
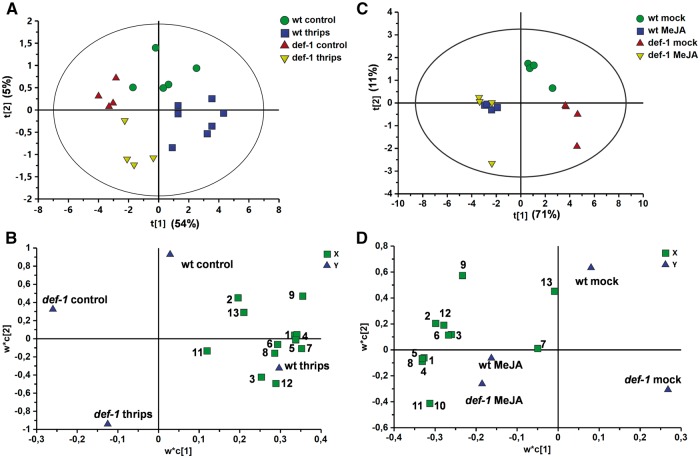
Analysis of the total production of leaf trichome-associated volatiles revealed that terpene content increased with thrips infestation treatment in wild-type plants (GLM: Wald χ2 = 8.23, P = 0.004 for infestation treatment; Wald χ2 = 24.27, P < 0.001 for plant genotype; Wald χ2 = 38.61, P = 0.312 for the interaction) (Fig. 5A). Though a small increase in terpene content was also detected in thrips-infested def-1 plants, this was not statistically significant when compared with non-infested plants. Among the induced compounds in leaf exudates of thrips-infested wild-type plants, a statistically significant increase in levels of α-pinene, δ-carene, α-phellandrene, α-terpinene, limonene and β-phellandrene, as well as a non-identified terpene compound, was detected [GLM followed by least significant difference (LSD) post-hoc tests, P ≤ 0.05] (Table 1). Myrcene and another non-identified terpene were also slightly induced in thrips-infested def-1 plants. To better differentiate production of induced volatiles among treatments, a supervised multivariate partial least squares discriminant analysis (PLS-DA) analysis was performed. The analysis resulted in a model with one significant principal component (PC) [model statistics: R2X = 0.55, R2Y = 0.28 and Q2 = 0.22; analysis of variance of the cross-validated residuals (CV-ANOVA), P = 0.006] that explained 54% of the variance (Fig. 6A). This PC significantly separated non-infested and infested def-1 plants from non-infested and thrips-infested wild-type plants, showing that production of practically all terpene compounds was reduced in def-1 (Fig. 6B).
Fig. 5.
Total terpene content (mean ± SEM, n = 4–6) in leaf exudates of non-infested and thrips-infested wild-type (wt) and def-1 plants (A) and mock-treated and MeJA-treated wt and def-1 plants (B). Samples were taken from the third/fourth youngest leaf at 12 d after the initial treatment. Different letters above bars denote significant differences among groups compared by Fisher’s LSD test at P ≤ 0.05.
Fig. 6.
PLS-DA of volatile compounds detected in leaf exudates of thrips-infested and non-infested wild-type (wt) and def-1 plants (A and B), and mock-treated and MeJA-treated wild-type (wt) and def-1 plants (C and D) at 12 d from initial treatment. Score plot (A and C) and loading plot (B and D) of the first two principal components (PCs) with the explained variance in parentheses. The second PC was not statistically significant and is only shown for representational purposes. The ellipse in (A) and (C) defines the Hotelling’s T2 confidence region (95%). The numbers in (B) and (D) represent: 1, α-pinene; 2, p-cymene; 3, myrcene; 4, δ-carene; 5, α-phellandrene; 6, α-terpinene; 7, limonene; 8, β-phellandrene; 9, trans-ocimene; 10, γ-terpinene; 11, terpinolene; 12, unknown; and 13, β-caryophyllene.
Exogenous application of MeJA significantly increased total terpene production in leaf exudates of wild-type and def-1 plants (GLM: Wald χ2 = 494.5, P < 0.001 for hormone treatment; Wald χ2 = 24.31, P < 0.001 for plant genotype; Wald χ2 = 42.51, P < 0.001 for the interaction) (Fig. 5B). Total terpene content in mock-treated def-1 plants was significantly lower than in mock-treated wild-type plants. Hence, larger differences in the induced production of terpenes were detected between mock-treated and MeJA-treated def-1 plants compared with the wild type, as shown by the significant interaction between genotype and treatment. Overall, a 4.4- and 15-fold increase in terpene content was detected in MeJA-treated wild-type and def-1 leaf exudates, respectively. Based on a 2.5- and 8.5-fold increase in type-VI leaf glandular trichome densities observed after MeJA treatment in wild-type and def-1 plants, respectively, a 2-fold induction of terpene production per trichome was estimated for both genotypes. In addition, some compounds (i.e. γ-terpinene and terpinolene) were only detected in wild-type and def-1 plants after MeJA application (Table 1). The PLS-DA analysis generated a model with one PC explaining 71% of the variance (model statistics: R2X = 0.71, R2Y = 0.321 and Q2 = 0.273; CV-ANOVA, P = 0.047), that clearly separated volatile patterns of mock-treated and MeJA-treated plants (Fig. 6C). This model also highlighted differences between mock-treated wild-type and def-1 plants, and between MeJA-treated wild-type and def-1 plants. The loading plot showed that nearly all terpene compounds were highly induced in MeJA-treated wild-type and def-1 plants (Fig. 6D).
Higher silver damage symptoms correlate positively with increased trichome-associated volatile accumulation in wild-type plants
To investigate further whether the magnitude of the thrips-mediated induction of type-VI trichomes and their associated volatiles is determined by the feeding damage thrips cause in the plant, a positive gradient of silver damage symptoms was generated by infesting wild-type tomato plants with 0, 20, 40 and 60 thrips (Fig. 7). There was a positive correlation, near to statistically significant, between silver damage symptoms and type-VI trichome density (one-tailed Pearson, r = 0.338, n = 24, P = 0.053) (Fig. 7A). A significant and positive correlation between silver damage symptoms and the total volatile content in leaf exudates was also observed (one-tailed Pearson, r = 0.537, n = 24, P = 0.003) (Fig. 7B). Type-VI trichome densities and the total volatile content in leaf exudates were also positively correlated (one-tailed Pearson, r = 0.577, n = 24, P = 0.002) (Fig. 7C). Production of most of the terpene compounds detected in the leaf exudates correlated positively with silver damage levels and type-VI trichome densities (Supplementary Table S1).
Fig. 7.
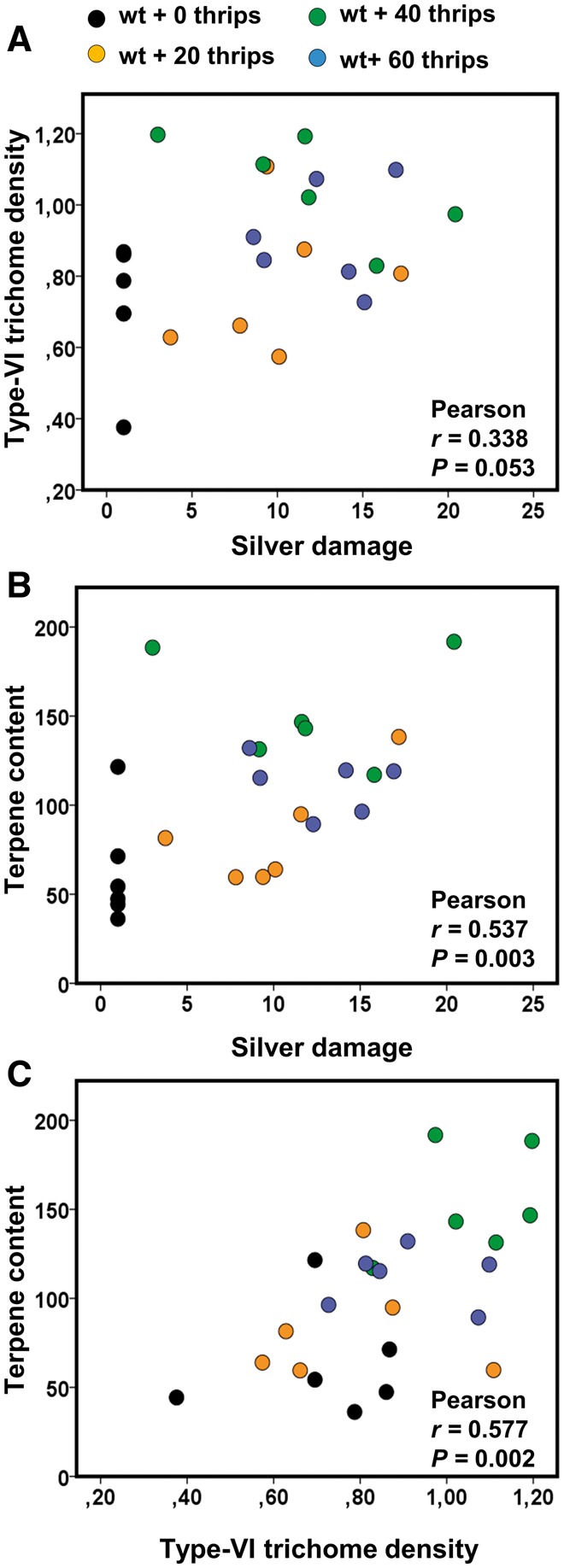
Scatter plots depicting the relationship between silver damage symptoms [square root (x + 1)-transformed data] and type-VI trichome density (log-transformed data) (A), silver damage symptoms [square root (x + 1)-transformed data] and total terpene content (µg g–1 FW) in leaf exudates (B), and type-VI trichome density (log-transformed data) and total terpene content in leaf exudates (C). Data were obtained from wild-type plants infested with 0, 20, 40 and 60 thrips (n = 6 plants per treatment) at 12 d after thrips infestation.
Discussion
Our study showed that previous infestation of S. lycopersicum tomato plants with F. occidentalis negatively affected host plant acceptance by conspecifics. We demonstrated that these plant-mediated intraspecific interactions for thrips can be determined by activation of JA defenses in tomato. Hence, in dual-choice assays, thrips showed a clear preference for non-infested over thrips-infested leaf discs taken from wild-type plants, while they did not discriminate between leaf discs taken from non-infested and thrips-infested JA-deficient def-1 plants. Moreover, when JA-associated defenses were artificially induced in both genotypes, by means of exogenous application of the JA volatile methyl ester MeJA, thrips showed a stronger preference for non-induced leaf discs. This response is not exclusive for thrips, and many lepidopteran species also avoid plants damaged by conspecifics (Sato et al. 1999, De Moraes et al. 2001, Kessler and Baldwin 2001). Previous studies have described the negative impact of induction of plant defenses on host-plant selection, feeding and survival of F. occidentalis (Agrawal et al. 1999, Agrawal and Corfel 2000, Delphia et al. 2007, Abe et al. 2009, De Puysseleyr et al. 2011, Kammerhofer et al. 2015). However, until now, characterization of the role of JA defenses induced directly by thrips in the subsequent colonization by conspecifics had not been investigated. We showed that the JA-associated marker WIPI-II was induced after thrips infestation in wild-type plants but not in def-1. Conversely, no induction of the SA-associated marker, PR-P6, was observed in both genotypes. Accordingly, JA has been reported as the principal signaling pathway triggered by thrips in Arabidopsis (De Vos et al. 2005, Abe et al. 2008) and tomato (Li et al. 2002, Kawazu et al. 2012). Our results further suggest that activation of the ET signaling pathway by thrips in wild-type and def-1 plants might not be crucial for tomato resistance to thrips. In line with this, Abe et al. (2008) suggested the possible importance of JA, ET and SA signals in the response of Arabidopsis to thrips feeding. However, the role of the ET signaling pathway on thrips–tomato interactions needs further research.
We demonstrated that JA-induced defenses after thrips infestation in wild-type tomato plants resulted in enhanced type-VI leaf trichome-associated defenses, a response detected 12 d after initial infestation. In tomato, JA has been proposed as a central regulator of trichome density and biochemistry (Li et al. 2004, Boughton et al. 2005, van Schie et al. 2007). In accordance with this, def-1 plants, impaired in JA-induced defense responses, did not increase trichomes upon thrips herbivory. In contrast, exogenous application of MeJA increased type-VI trichome densities and total number per leaflet in wild-type and def-1 plants. Induction of leaf trichome densities is a common phenotype described in distinct and unrelated plant species when alterations in biotic and abiotic conditions take place. In particular, changes in temperature or light conditions (Kennedy 2003), mechanical damage (Dalin et al. 2008), herbivory (Traw and Dawson 2002, Dalin and Bjorkman 2003), defoliation (Abdala-Roberts et al. 2005), or treatment with larval oral secretions (Tian et al. 2012a) all have been observed to give rise to higher trichome densities on newly emerged leaves. Different features of the plant leaf surface can determine the success of herbivore colonization (Howe and Jander 2008). Changes in physical and chemical leaf properties can, therefore, provide valuable information about potential mechanisms responsible for increased resistance to subsequent herbivore attack. In this sense, some studies have reported that herbivore-induced plants with increased leaf trichome densities showed less damage by insects than non-infested plants (Baur et al. 1991, Agrawal 1999, Dalin and Björkman 2003).
In addition, we provide evidence that herbivorous arthropods can not only induce trichome densities in infested plants, but can also alter the overall content of the leaf trichome-associated volatiles. Many studies have described the emission of plant volatiles after herbivory in tomato (Wei et al. 2012). However, the volatiles measured in the headspace of non-infested or infested plants might differ from those measured in leaf exudates. Though a great part of the emitted plant volatiles in cultivated tomatoes are produced by glandular trichomes (Kessler and Baldwin 2001, Kant et al. 2009, Kang et al. 2010a), other compounds such as methyl salicylate are produced by non-trichome tissues (van Schie et al. 2007). Kang et al. (2010a) reported that in od-2 tomato mutants, defective in trichome-derived volatiles, plant wounding induced the emission of the green leaf volatiles hexanal and cis-3-hexanal. In addition, differences in terpene composition and production between leaf and stem trichomes were reported for tomato (Tian et al. 2012b). Our data show that after thrips infestation wild-type plants produced more leaf trichome-associated volatiles, while damaged def-1 plants did not significantly increase their overall production. In particular, thrips-infested wild-type plants accumulated more α-pinene, δ-carene, α-phellandrene, α-terpinene, limonene, β-phellandrene and a non-identified compound per leaflet than non-infested plants. Some of these compounds (i.e. α-pinene, δ-carene and β-phellandrene) have been reported to be emitted by wild-type (Castlemart) plants infested with Spodoptera exigua (Thaler et al. 2002), suggesting a common response to that described for thrips here. A slight but significant increase in the levels of myrcene and a non-identified compound was also observed in thrips-infested def-1 plants. Thaler et al. (2002) also described herbivore-mediated induction of VOCs for the def-1 genotype. According to those authors, an increase in emitted volatile compounds was detected in S. exigua-infested def-1 plants, but at a much lower magnitude (i.e. 34% less monoterpenes and 51% less sesquiterpenes) when compared with infested wild-type plants. Hence, even though def-1 is able to respond slightly to herbivory, these responses were reported as insufficient to mount effective direct and indirect plant defenses against different arthropod herbivores (Howe et al. 1996, Thaler et al. 2002, Ament et al. 2004). Accordingly, our results showed that the def-1 genotype was more susceptible to thrips than the wild type. In this sense, we observed that not only inducible but also constitutive trichome-associated chemical defenses were diminished in def-1 plants. Total terpene content in the leaf exudates of non-infested and mock-treated def-1 plants was significantly lower compared with the wild type. This might be explained by a lower number of type-VI leaf glandular trichomes in def-1 (Fig. 4). This observation agrees with Peiffer et al. (2009) where nearly 65% lower type-VI glandular trichome density in def-1 compared with the wild-type genotype was observed. Levels of constitutive emitted volatiles in def-1, however, were reported to be similar to those detected in the wild type (Thaler et al. 2002), which might be explained by the compensatory volatile emission from other plant tissues.
Application of exogenous MeJA on wild-type and def-1 plants triggered qualitatively and quantitatively different trichome-associated defense responses when compared with those observed in thrips-infested wild-type plants. Though thrips infestation altered trichome densities in the wild-type genotype, we concluded that it might not influence the terpene production per trichome, as the ratio of increased trichome densities and volatile content was determined as 1 : 1. In contrast, in addition to higher type-VI leaf glandular trichome densities, higher terpene production per leaflet was observed in MeJA-treated wild-type and def-1 plants, suggesting an increased production per trichome. Moreover, some compounds (i.e. p-cymene, γ-terpinene and terpinolene) were only detected or induced by MeJA. Differences in the chemical profiles and the magnitude of the induction of trichome-associated defenses in thrips-infested and MeJA-treated wild-type plants might rely on different foliar JA levels, i.e. associated with variable thrips damage intensities, produced in the plant after the infestation. To test this, we generated a gradient in silver damage symptoms by infesting wild-type plants with different densities of thrips. Our results indeed showed a positive correlation between the silver damage symptoms and type-VI trichome densities, as well as with the content of terpene compounds in the leaf exudates of wild-type plants. The volatile content in the leaf exudates was also positively correlated with type-VI trichome density. As thrips infestation activates JA signaling in tomato, we hypothesize that higher thrips feeding damage might have induced increased production of this phytohormone and, consequently, also the production of type-VI trichomes and their associated volatiles. For instance, a direct positive relationship between endogenous JA levels and both sesquiterpene and indole volatile emission has been demonstrated in maize (Zea mays) (Schmelz et al. 2003). In the same study, Schmelz et al. (2003) also showed that JA production was positively correlated with infestation levels of maize plants with S. exigua caterpillars. Similarly, Thaler et al. (2001) described that artificially JA-mediated induction of defensive proteins in tomato can respond in a positive dose-dependent manner. This might also explain the differences in the induction of WIPI-II expression, controlled by JA signaling (Constabel et al. 1995, Alba et al. 2015), between thrips-infested and hormone-treated wild-type plants. Alternatively, induction of ET defense signaling in thrips-infested plants might have influenced induced JA-associated responses. Hence, ET can act antagonistically to suppress JA-induced expression of nicotine biosynthesis in tobacco (Rojo et al. 1999, Shoji et al. 2000).
Interestingly, our results also showed that when offered a choice between leaf discs from mock-treated and MeJA-treated wild-type or def-1 plants, a larger percentage of thrips preferred non-induced leaf discs compared with the dual-choice assays using leaf discs from control and thrips-infested wild-type plants. A higher production of trichome-associated volatiles in MeJA-treated plants might explain these differences. Changes in amounts of VOCs produced by leaves can greatly affect phytophagous insects, since they exploit these cues to take foraging decisions prior to and after contact with potential host plants (Bruce et al. 2005). In particular, terpenes, one of the largest class of volatiles identified in plants (Pickersky et al. 2006), play an important role in trichome-mediated resistance of tomato wild species to different arthropod herbivores (Freitas et al. 2002, De Azevedo et al. 2003, Bleeker et al. 2012). In cultivated species, glandular leaf trichomes actively produce mono- and sesquiterpenes, and their absence and/or altered chemistry can result in higher susceptibilities to arthropod herbivores (Kang et al. 2010a, Kang et al. 2010b, Tian et al. 2012b). Thrips are reported to perceive and react in response to plant volatiles, that in turn can act as feeding and oviposition deterrents or affect thrips post-landing behavior (Koschier et al. 2002, Koschier et al. 2007, Allsop et al. 2014). Changes in leaf trichome-associated volatiles in thrips-infested and MeJA-treated wild-type plants might explain the changes in the host plant acceptance by conspecifics described in our study. In this sense, α-pinene and p-cymene, both compounds reported here as induced after thrips or MeJA induction in wild-type plants, are reported as toxic agents for F. occidentalis and Thrips tabaci (Koshier et al. 2002, Janmaat et al. 2002). Interestingly, p-cymene, α-terpinene and α-phellandrene, shown as induced in the present work, have been described as potent repellent agents against whiteflies (Bleeker et al. 2009). On the other hand, repellence properties might be influenced by the composition and concentration of the overall terpene mixture present (Bleeker et al. 2009). Whether the blend or levels of individual compounds detected in thrips- or MeJA-induced leaflets might exert repellent or toxic effects on F. occidentalis will require further tests. Additional studies will also be needed to determine the contribution of the induced trichome-associated defenses to the emitted plant volatiles upon thrips infestation in tomato.
In conclusion, we demonstrated that thrips-mediated induction of JA-associated defenses in tomato negatively affects plant host acceptance by F. occidentalis conspecifics. These plant-mediated intraspecific interactions might be in part explained by the induction of trichome-associated defenses, controlled by the activation of the JA signaling pathway. We describe here that trichome-derived leaf volatile production can be modulated by thrips infestation in tomato. The magnitude and/or type of this induction, as shown by the stronger plant response and different chemical profiles of leaf trichomes after MeJA hormone treatment or increasing thrips infestation, might influence plant attractiveness to herbivores.
Materials and Methods
Plant material and insects
Tomato seeds (Solanum lycopersicum Mill cv ‘Castlemart’ and the jasmonate-deficient mutant def-1, kindly provided by Professor Gregg Howe, Michigan State University) were sown in plastic trays filled with potting soil in a climate room provided with 113.6 µmol m2 s–1 photosynthetically active radiation (PAR), a photoperiod of 16 h light/8 h dark, 20°C and 70% relative humidity (RH). Fifteen days after germination, plantlets were transplanted to 11 cm diameter plastic pots. Western flower thrips were obtained from a colony reared on chrysanthemum flowers maintained in a climate room at 25°C and 70% RH.
Plant treatments
Four-week old Castlemart (wild-type) and def-1 plants were individually placed into thrips-proof cages consisting of a plastic cylinder (80 cm height, 20 cm diameter) closed at one end with a lid made of thrips-proof gauze (Leiss et al. 2009). Seven plants per genotype were then subjected to the following treatments: (i) thrips infestation; (ii) no thrips; (iii) MeJA; or (iv) mock treatment. For thrips infestation, 20 adult thrips (18 females and two males) per plant were released inside the cage. The hormone treatment consisted of spraying plants with 7.5 mM MeJA (Sigma-Aldrich) in 0.8% ethanol aqueous solution until the point of run-off as described by Boughton et al. (2005). Mock treatment with 0.8% ethanol aqueous solution was used as control. All cages were randomly placed in a climate room provided with 113.6 µmol m–2 s–1 PAR, a photoperiod of 16 h light/8 h dark, 25°C and 70% RH. After 12 d, plants were sampled for measurements and chemical analysis. Thrips feeding damage (i.e. silver damage) was evaluated for the whole plant and expressed as mm2 of total damaged leaf area (Leiss et al. 2009). Silver damage, which thrips cause by sucking the epidermal/mesophyll plant cell content, was visually scored under a stereomicroscope by determining the area of the local necrosis, appearing as scars, in the leaves. Leaflets from the third/fourth youngest and fully expanded leaf were sampled for thrips preference bioassays, trichome density and total trichome number per leaflets, terpene content in leaf exudates, epidermal cell number and gene expression analyses. This leaf was chosen because it was susceptible to trichome induction when the treatments were applied (Traw and Dawson 2002). Because F. occidentalis preferentially feeds on old tomato leaves (Mirnezhad et al. 2010), young leaves did not show silver damage symptoms, eggs or larvae at the time of sampling.
To determine whether differences in the magnitude of the induction of type-VI trichome density and production of terpenes are influenced by distinct levels of thrips damage, an additional experiment with the wild-type genotype was performed. Three different densities of thrips infestation, i.e. 20, 40 and 60 thrips, were applied per plant (i.e. six plants per treatment) using the same thrips-proof cages described above. Silver damage symptoms, type-VI trichome density in adaxial leaf sides and volatile content in leaf exudates were measured at 12 d after thrips release. Non-infested plants were used as controls.
Leaf disc dual-choice assay
A dual-choice assay (Leiss et al. 2009) was used to test thrips preference for leaf discs taken from non-infested vs. thrips-infested and mock-treated vs. MeJA-treated wild-type or def-1 plants. This bioassay was repeated twice in two independent experiments. Silver damage symptoms were measured in thrips-infested plants before sampling for the leaf disc bioassays in the two experiments. Excised undamaged leaflets from the third/fourth youngest leaf were used. Leaf discs, each corresponding to an individual plant, with a diameter of 20 mm were punched from tomato leaves and placed on a thin layer of 1% water agar in a 90 mm diameter Petri dish. For each pairwise test, 5–7 replicates, i.e. Petri dishes, were evaluated. Ten starved female F. occidentalis adults were anesthetized briefly with CO2 and placed on a small filter paper positioned between the discs. The Petri dishes were sealed with parafilm and placed in a climate room at 25°C and 16 h light/8 h dark light regime. The number of thrips on each leaf disc was recorded at 0.5, 1, 2, 3 and 4 h after thrips release. The number of thrips recorded in each leaf disc at the different time points were averaged and shown as the percentage of thrips that made a choice in that period of time.
Trichome density and total number
Type-VI trichome density was measured on the adaxial and abaxial surfaces of leaflets taken from the third/fourth youngest and fully expanded leaf. A stereomicroscope Leica MZ16 (Leica Microsystems) equipped with a Leica DFC420 digital camera was used to take pictures of an area of 12 mm2 at both leaf sides of the main vein to generate a mean of these two measurements. Type-VI trichomes were counted using the ImageJ software (http://imagej.nih.gov/ij/) and density was expressed as number of type-VI trichomes per mm2. Thereafter, the leaflet area’s were determined by scanning and analysis of scanned pictures using the ImageJ software. Estimations of the total number of type-VI trichomes per leaflet were obtained by multiplying trichome density (No. mm–2) per leaf area in mm2.
Terpene analysis
Terpene production by type-VI glandular trichomes was analyzed in leaf exudates collected from two leaflets belonging to the same leaf used for trichome density measurements. Before extraction, fresh weight was measured. Leaf exudates were obtained by dipping the leaf tissue in 2 ml of pentane containing 10 µg of benzyl acetate as internal standard (Schilmiller et al. 2009, Kang et al. 2010a, Kang et al. 2010b, Sallaud et al. 2012). Following an incubation period of 2 min with gentle shaking, the leaflets were removed. A 1 µl aliquot from the resulting pentane leaf extract was injected into an Agilent model 7890 gas chromatograph fitted with a 5975C inert XL MSD Triple Axis Detector using a split ratio of 20 : 1. Injector temperature was 280°C. The initial column (30 m × 0.25 mm, 0.25 µm film thickness, DB-5MS, Agilent Technologies) temperature was set at 40°C, then ramped to 150°C at 15°C min–1 and finally to 280°C at 3°C min–1. The helium carrier gas flow was 1.6 ml min–1. Terpenes were identified by comparison with authentic standards when possible or by comparison with retention times and spectral information available in Agilent GC/MSD ChemStation. Compounds were quantified on the basis of the internal standard procedure. Calibration curves of known concentrations (five data points in the range of 0.5–60 µg) of synthetic external standards were generated to calculate the internal response factor (IRF). For this, α-pinene and β-caryophyllene (Sigma-Aldrich) were used as external standards to determine the IRF of monoterpenes and sesquiterpenes, respectively. Calculations of the terpene concentrations were based on the equation: amount of specific compound = (amount IS × area SC × IRF)/area IS, where IS corresponds to the internal standard, SC to the specific compound of interest and IRF to the internal response factor. Terpene content was expressed as µg g–1 FW.
Measurement of epidermal cell number
To determine whether changes in trichome density and number were related to changes in cell size, epidermal cell density was determined in adaxial leaf sides by using an imprint technique (Kirkham et al. 1972). First, leaflets were scanned and leaf area was determined using the ImageJ software. Next, a clear nail polish was applied to the adaxial leaflet surface and, after the polish had dried, strips with cell impressions were peeled off and photographed on a light microscope (B-350, OPTIKA) at × 400 magnification. Cell number was counted using the ImageJ software and expressed as number of epidermal cells per mm2.
Gene expression analysis
Total RNA was isolated as described in Verdonk et al. (2003) and treated with DNase (Ambion). cDNA was synthesized from 4 µg of total RNA using M-MuLV Reverse Transcriptase (Fermentas) in a 20 µl reaction. Quantitative reverse transcription–PCR (qRT–PCR) was performed in CFX96™ Optics Module (BIO-RAD) using iQ™ SYBR® Green Supermix (BIO-RAD). PCRs of 20 µl contained 0.25 µM of each primer and 1 µl of cDNA. The cycling program was set to 5 min at 50°C, 2 min at 95°C, 40 cycles of 15 s at 95°C and 1 min at 60°C, followed by a melting curve analysis. Three biological replicates with two technical qRT–PCR replicates (i.e. individual plants) were analyzed per treatment. Actin was used as a reference gene. The normalized expression (NE) data were calculated with the 2−ΔΔCt method. NE values were scaled to the lowest average NE within the plot, which was set to 1. Transcript levels of the JA marker gene WIPI-II, the SA marker gene PR-P6 (Alba et al. 2015) and the ET-responsive marker gene SlERF1b (Nambeesan et al. 2012) were analyzed. The gene-specifc primers used for the qRT–PCRs are shown in Supplementary Methods S1.
Statistical analysis
All data were first analyzed using Levene and Kolmogorov–Smirnov tests to determine the heteroscedasticity of variance and normality, respectively. Differences in silver damage between wild-type and def-1 thrips-infested plants were analyzed by unpaired Student’s t-tests. Wilcoxon matched-pairs signed-rank tests were used to assess significant differences in thrips preference between leaf discs taken from infested vs. control plants, and between leaf discs taken from MeJA-treated vs. mock-treated plants for each plant genotype. The number of thrips settled on one of the two leaf discs at 0.5, 1, 2, 3 and 4 h after release was pooled before analysis. Effect of treatment (thrips infestation or hormone application), genotype and their interaction on trichome density, number of trichomes per leaflet, epidermal cell density, total terpene production per g of FW, and gene expression was analyzed by GLMs, using the linear distribution and identity link function, followed by Fisher’s LSD post-hoc test. Data from trichome densities, total terpene production and gene expression analysis were log transformed prior to analysis when needed. Pearson tests were performed to analyze correlation between silver damage symptoms, type-VI trichome density and volatile content in wild-type non-infested plants and plants infested with 20, 40 and 60 thrips. Pearson and Spearman tests were used to analyze correlation between content of individual terpene compounds, silver damage and type-VI trichome density. Statistical analyses were performed by using the SPSS software package (version 21; SPSS Inc.). Patterns of volatiles in thrips-infested or hormone-treated plants were subjected to principal component analysis (PCA) and PLS-DA using the software SIMCA-P version 13 (Umetrics). Data were log transformed when needed (the constant 0.0001 was added to provide non-detectable components with a small non-zero value), mean centered and scaled to unit variance prior to PCA and PLS-DA (Eriksson et al. 2001). Statistical significance of PLS-DA models was tested by using the CV-ANOVA method (Eriksson et al. 2008). Additionally, data from identified volatiles were tested for significant differences among plant treatments (thrips infestation or hormone treatments) by GLM and LSD post-hoc tests. For this analysis, data from mock- and MeJA-treated plants were log transformed.
Supplementary data
Supplementary data are available at PCP online.
Funding
This work was supported by the STW Perspective program ‘Green Defense against Pests’ (GAP) [Ref.13553; we thank the companies involved in the GAP project: Rijk Zwaan, Duemmen Orange, Dekker Chrysanten, Fides and Incotec for financial support].
Supplementary Material
Acknowledgments
We thank María-José Rodríguez-López and Erica Wilson for assistance with plant damage evaluation and volatile measurements, respectively.
Glossary
Abbreviations
- ANOVA
analysis of variance
- CV-ANOVA
ANOVA of the cross-validated residuals
- ET
ethylene
- GLM
generalized linear model
- IRF
internal response factor
- IS
internal standard
- JA
jasmonic acid
- LSD
least significant difference
- MeJA
methyl jasmonate
- NE
normalized expression
- PAR
photosynthetically active radiation
- PC
principal component
- PCA
principal component analysis
- PLS-DA
partial least square discriminant analysis
- PR-P6
pathogenesis-related protein 6
- qRT–PCR
quantitative reverse transcription–PCR
- RH
relative humidity
- SA
salicylic acid
- SC
specific compound
- SlERF1b
Solanum lycopersicum ethylene-responsive factor
- VOC
volatile organic compound
- WIPI-II
wound inducible proteinase inhibitor II
Disclosures
The authors have no conflicts of interest to declare.
References
- Abdala-Roberts L., Parra-Tabla V. (2005) Artificial defoliation induces trichome production in the tropical shrub Cnidoscolus aconitifolius (Euphorbiaceae) 1. Biotropica 37: 251–257. [Google Scholar]
- Abe H., Ohnishi J., Narusaka M., Seo S., Narusaka Y., Tsuda S. et al. (2008) Function of jasmonate in response and tolerance of Arabidopsis to thrip feeding. Plant Cell Physiol. 49: 68–80. [DOI] [PubMed] [Google Scholar]
- Abe H., Shimoda T., Ohnishi J., Kugimiya S., Narusaka M., Seo S. et al. (2009) Jasmonate-dependent plant defense restricts thrips performance and preference. BMC Plant Biol. 9: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A.A. (1999) Induced responses to herbivory in wild radish: effects on several herbivores and plant fitness. Ecology 80: 1713–1723. [Google Scholar]
- Agrawal A.A., Colfer R.G. (2000) Consequences of thrips-infested plants for attraction of conspecifics and parasitoids. Ecol. Entomol. 25: 493–496. [Google Scholar]
- Agrawal A.A., Kobayashi C., Thaler J.S. (1999) Influence of prey availability and induced host-plant resistance on omnivory by western flower thrips. Ecology 80: 518–523. [Google Scholar]
- Alba J.M., Schimmel B.C., Glas J.J., Ataide L., Pappas M.L., Villarroel C.A. et al. (2015) Spider mites suppress tomato defenses downstream of jasmonate and salicylate independently of hormonal crosstalk. New Phytol. 205: 828–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsopp E., Prinsloo G., Smart L., Dewhirst S. (2014) Methyl salicylate, thymol and carvacrol as oviposition deterrents for Frankliniella occidentalis (Pergande) on plum blossoms. Arthropod Plant Interact. 8: 421–427. [Google Scholar]
- Ament K., Kant M.R., Sabelis M.W., Haring M.A., Schuurink R.C. (2004) Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol. 135: 2025–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur R., Binder S., Benz G. (1991) Nonglandular leaf trichomes as short-term inducible defense of the grey alder, Alnus incana (L.), against the chrysomelid beetle, Agelastica alni L. Oecologia 87: 219–226. [DOI] [PubMed] [Google Scholar]
- Besser K., Harper A., Welsby N., Schauvinhold I., Slocombe S., Li Y. et al. (2009) Divergent regulation of terpenoid metabolism in the trichomes of wild and cultivated tomato species. Plant Physiol. 149: 499–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleeker P.M., Diergaarde P.J., Ament K., Guerra J., Weidner M., Schütz S. et al. (2009) The role of specific tomato volatiles in tomato–whitefly interaction. Plant Physiol. 151: 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleeker P.M., Mirabella R., Diergaarde P.J., VanDoorn A., Tissier A., Kant M.R. et al. (2012) Improved herbivore resistance in cultivated tomato with the sesquiterpene biosynthetic pathway from a wild relative. Proc. Natl. Acad. Sci. USA 109: 20124–20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughton A.J., Hoover K., Felton G.W. (2005) Methyl jasmonate application induces increased densities of glandular trichomes on tomato, Lycopersicon esculentum. J. Chem. Ecol. 31: 2211–2216. [DOI] [PubMed] [Google Scholar]
- Bruce T.J., Wadhams L.J., Woodcock C.M. (2005) Insect host location: a volatile situation. Trends Plant. Sci. 10: 269–274. [DOI] [PubMed] [Google Scholar]
- Chen H., Jones A.D., Howe G.A. (2006) Constitutive activation of the jasmonate signaling pathway enhances the production of secondary metabolites in tomato. FEBS Lett. 580: 2540–2546. [DOI] [PubMed] [Google Scholar]
- Constabel C.P., Bergey D.R., Ryan C.A. (1995) Systemin activates synthesis of wound-inducible tomato leaf polyphenol oxidase via the octadecanoid defense signaling pathway. Proc. Natl. Acad. Sci. USA 92: 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalin P., Ågren J., Björkman C., Huttunen P., Kärkkäinen K. (2008) Leaf trichome formation and plant resistance to herbivory. In Induced Plant Resistance to Herbivory. Edited by Schaller A. pp. 89–105. Springer, Berlin. [Google Scholar]
- Dalin P., Björkman C. (2003) Adult beetle grazing induces willow trichome defence against subsequent larval feeding. Oecologia 134: 112–118. [DOI] [PubMed] [Google Scholar]
- de Azevedo S.M., Faria M.V., Maluf W.R., De Oliveira A.C.B., de Freitas J.A. (2003) Zingiberene-mediated resistance to the South American tomato pinworm derived from Lycopersicon hirsutum var. hirsutum. Euphytica 134: 347–351. [Google Scholar]
- De Moraes C.M., Mescher M.C., Tumlinson J.H. (2001) Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 410: 577–580. [DOI] [PubMed] [Google Scholar]
- De Puysseleyr V., Höfte M., De Clercq P. (2011) Ovipositing Orius laevigatus increase tomato resistance against Frankliniella occidentalis feeding by inducing the wound response. Arthropod Plant Interact. 5: 71–80. [Google Scholar]
- De Vos M., Van Oosten V.R., Van Poecke R.M., Van Pelt J.A., Pozo M.J., Mueller M.J. et al. (2005) Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol. Plant. Microbe Interact. 18: 923–937. [DOI] [PubMed] [Google Scholar]
- Delphia C.M., Mescher M.C., De Moraes C.M. (2007) Induction of plant volatiles by herbivores with different feeding habits and the effects of induced defenses on host-plant selection by thrips. J. Chem. Ecol. 33: 997–1012. [DOI] [PubMed] [Google Scholar]
- Denno R.F., Kaplan I. (2007) Plant-mediated interactions in herbivorous insects: mechanisms, symmetry, and challenging the paradigms of competition past. In Ecological Communities: Plant Mediation in Indirect Interaction Webs. Edited by Ohgushi T., Craig P., Price P.W. pp. 19–50. Cambridge University Press, Cambridge. [Google Scholar]
- Dicke M., Loon J.J. (2000) Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol. Exp. Appl. 97: 237–249. [Google Scholar]
- Erb M., Robert C.A., Hibbard B.E., Turlings T.C. (2011) Sequence of arrival determines plant-mediated interactions between herbivores. J. Ecol. 99: 7–15. [Google Scholar]
- Eriksson L., Byrne T., Johansson E., Trygg J., Vikström C. (2013) Multi- and Megavariate Data Analysis Basic Principles and Applications. Umetrics Academy. [Google Scholar]
- Eriksson L., Trygg J., Wold S. (2008) CV-ANOVA for significance testing of PLS and OPLS® models. J. Chemom. 22: 594–600. [Google Scholar]
- Farmer E.E., Ryan C.A. (1990) Interplant communication: airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc. Natl. Acad. Sci. USA 87: 7713–7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler J.H., Narváez-Vásquez J., Aromdee D.N., Pautot V., Holzer F.M., Walling L.L. (2009) Leucine aminopeptidase regulates defense and wound signaling in tomato downstream of jasmonic acid. Plant Cell 21: 1239–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas J.A., Maluf W.R., das Graças Cardoso M., Gomes L.A., Bearzotti E. (2002) Inheritance of foliar zingiberene contents and their relationship to trichome densities and whitefly resistance in tomatoes. Euphytica 127: 275–287. [Google Scholar]
- Glas J.J., Alba J.M., Simoni S., Villarroel C.A., Stoops M., Schimmel B.C. et al. (2014) Defense suppression benefits herbivores that have a monopoly on their feeding site but can backfire within natural communities. BMC Biol. 12: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glas J.J., Schimmel B.C., Alba J.M., Escobar-Bravo R., Schuurink R.C., Kant M.R. (2012) Plant glandular trichomes as targets for breeding or engineering of resistance to herbivores. Int. J. Mol. Sci. 13: 17077–17103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe G.A., Jander G. (2008) Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 59: 41–66. [DOI] [PubMed] [Google Scholar]
- Howe G.A., Lightner J., Ryan C. (1996) An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. Plant Cell 8: 2067–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmaat A.F., Kogel W.J.D., Woltering E.J. (2002) Enhanced fumigant toxicity of p-cymene against Frankliniella occidentalis by simultaneous application of elevated levels of carbon dioxide. Pest Manag. Sci. 58: 167–173. [DOI] [PubMed] [Google Scholar]
- Kammerhofer N., Egger B., Dobrev P., Vankova R., Hofmann J., Schausberger P. et al. (2015) Systemic above-and belowground cross talk: hormone-based responses triggered by Heterodera schachtii and shoot herbivores in Arabidopsis thaliana. J. Exp. Bot. 66: 7005–7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J.H., Liu G., Shi F., Jones A.D., Beaudry R.M., Howe G.A. (2010a) The tomato odorless-2 mutant is defective in trichome-based production of diverse specialized metabolites and broad-spectrum resistance to insect herbivores. Plant Physiol. 154: 262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J.H., McRoberts J., Shi F., Moreno J.E., Jones A.D., Howe G.A. (2014) The flavonoid biosynthetic enzyme chalcone isomerase modulates terpenoid production in glandular trichomes of tomato. Plant Physiol. 164: 1161–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J.H., Shi F., Jones A.D., Marks M.D., Howe G.A. (2010b) Distortion of trichome morphology by the hairless mutation of tomato affects leaf surface chemistry. J. Exp. Bot. 61: 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant M.R., Bleeker P.M., Van Wijk M., Schuurink R.C., Haring M.A. (2009) Plant volatiles in defence. Adv. Bot. Res. 51: 613–666. [Google Scholar]
- Karban R., Baldwin I. (1997) Induced Responses to Herbivory. University of Chicago Press; Chicago, IL. [Google Scholar]
- Karban R., Myers J.H. (1989) Induced plant responses to herbivory. Annu. Rev. Ecol. Syst. 20: 331–348. [Google Scholar]
- Kawazu K., Mochizuki A., Sato Y., Sugeno W., Murata M., Seo S. et al. (2012) Different expression profiles of jasmonic acid and salicylic acid inducible genes in the tomato plant against herbivores with various feeding modes. Arthropod Plant Interact. 6: 221–230. [Google Scholar]
- Keinänen M., Oldham N.J., Baldwin I.T. (2001) Rapid HPLC screening of jasmonate-induced increases in tobacco alkaloids, phenolics, and diterpene glyco sides in Nicotiana attenuata. J. Agric. Food Chem. 49: 3553–3558. [DOI] [PubMed] [Google Scholar]
- Kennedy G.G. (2003) Tomato, pests, parasitoids, and predators: tritrophic interactions involving the genus Lycopersicon. Annu. Rev. Entomol. 48: 51–72. [DOI] [PubMed] [Google Scholar]
- Kessler A., Baldwin I.T. (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291: 2141–2144. [DOI] [PubMed] [Google Scholar]
- Kirkham M.B., Gardner W., Gerloff G. (1972) Regulation of cell division and cell enlargement by turgor pressure. Plant Physiol. 49: 961–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koorneef A., Pieterse C.M.J. (2008) Cross talk in defense signaling. Plant Physiol. 146: 839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koschier E., Hoffmann D., Riefler J. (2007) Influence of salicylaldehyde and methyl salicylate on post-landing behaviour of Frankliniella occidentalis Pergande. J. Appl. Entomol. 131: 362–367. [Google Scholar]
- Koschier E.H., Sedy K.A., Novak J. (2002) Influence of plant volatiles on feeding damage caused by the onion thrips Thrips tabaci. Crop Prot. 21: 419–425. [Google Scholar]
- Leiss K.A., Choi Y.H., Abdel-Farid I.B., Verpoorte R., Klinkhamer P.G. (2009) NMR metabolomics of thrips (Frankliniella occidentalis) resistance in Senecio hybrids. J. Chem. Ecol. 35: 219–229. [DOI] [PubMed] [Google Scholar]
- Li C., Williams M.M., Loh Y.-T., Lee G.I., Howe G.A. (2002) Resistance of cultivated tomato to cell content-feeding herbivores is regulated by the octadecanoid-signaling pathway. Plant Physiol. 130: 494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Zhao Y., McCaig B.C., Wingerd B.A., Wang J., Whalon M.E. et al. (2004) The tomato homolog of CORONATINE-INSENSITIVE1 is required for the maternal control of seed maturation, jasmonate-signaled defense responses, and glandular trichome development. Plant Cell 16: 126–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightner J., Pearce G., Ryan C.A. (1993) Isolation of signaling mutants of tomato (Lycopersicon esculentum). Mol. Gen. Genet. 241: 595–601. [DOI] [PubMed] [Google Scholar]
- Maes L., Goossens A. (2010) Hormone-mediated promotion of trichome initiation in plants is conserved but utilizes species and trichome-specific regulatory mechanisms. Plant Signal. Behav. 5: 205–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell E.T., Kapteyn J., Schmidt A., Li C., Kang J.-H., Descour A. et al. (2011) Comparative functional genomic analysis of Solanum glandular trichome types. Plant Physiol. 155: 524–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambeesan S., AbuQamar S., Laluk K., Mattoo A.K., Mickelbart M.V., Ferruzzi M.G. et al. (2012) Polyamines attenuate ethylene-mediated defense responses to abrogate resistance to Botrytis cinerea in tomato. Plant Physiol. 158: 1034–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omer A., Granett J., Karban R., Villa E. (2001) Chemically-induced resistance against multiple pests in cotton. Int. J. Pest Manage. 47: 49–54. [Google Scholar]
- Peiffer M., Tooker J.F., Luthe D.S., Felton G.W. (2009) Plants on early alert: glandular trichomes as sensors for insect herbivores. New Phytol. 184: 644–656. [DOI] [PubMed] [Google Scholar]
- Pichersky E., Noel J.P., Dudareva N. (2006) Biosynthesis of plant volatiles: nature’s diversity and ingenuity. Science 311: 808–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelman E.H., Broekgaarden C., Van Loon J.J., Dicke M. (2008) Early season herbivore differentially affects plant defence responses to subsequently colonizing herbivores and their abundance in the field. Mol. Ecol. 17: 3352–3365. [DOI] [PubMed] [Google Scholar]
- Reitz S.R. (2009) Biology and ecology of the western flower thrips (Thysanoptera: Thripidae): the making of a pest. Fla. Entomol. 92: 7–13. [Google Scholar]
- Robert C.A., Erb M., Duployer M., Zwahlen C., Doyen G.R., Turlings T.C. (2012) Herbivore-induced plant volatiles mediate host selection by a root herbivore. New Phytol. 194: 1061–1069. [DOI] [PubMed] [Google Scholar]
- Rojo E., León J., Sánchez-Serrano J.J. (1999) Cross-talk between wound signalling pathways determines local versus systemic gene expression in Arabidopsis thaliana. Plant J. 20: 135–142. [DOI] [PubMed] [Google Scholar]
- Sallaud C., Giacalone C., Töpfer R., Goepfert S., Bakaher N., Rösti S. et al. (2012) Characterization of two genes for the biosynthesis of the labdane diterpene Z-abienol in tobacco (Nicotiana tabacum) glandular trichomes. Plant J. 72: 1–17. [DOI] [PubMed] [Google Scholar]
- Sato Y., Yano S., Takabayashi J., Ohsaki N. (1999) Pieris rapae (Lepidoptera: Pieridae) females avoid oviposition on Rorippa indica plants infested by conspecific larvae. Appl. Entomol. Zool. 34: 333–337. [Google Scholar]
- Schilmiller A.L., Schauvinhold I., Larson M., Xu R., Charbonneau A.L., Schmidt A. et al. (2009) Monoterpenes in the glandular trichomes of tomato are synthesized from a neryl diphosphate precursor rather than geranyl diphosphate. Proc. Natl. Acad. Sci. USA 106: 10865–10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz E.A., Alborn H.T., Banchio E., Tumlinson J.H. (2003) Quantitative relationships between induced jasmonic acid levels and volatile emission in Zea mays during Spodoptera exigua herbivory. Planta 216: 665–673. [DOI] [PubMed] [Google Scholar]
- Shoji T., Nakajima K., Hashimoto T. (2000) Ethylene suppresses jasmonate-induced gene expression in nicotine biosynthesis. Plant Cell Physiol. 41: 1072–1076. [DOI] [PubMed] [Google Scholar]
- Thaler J.S., Farag M.A., Paré P.W., Dicke M. (2002) Jasmonate-deficient plants have reduced direct and indirect defences against herbivores. Ecol. Lett. 5: 764–774. [Google Scholar]
- Thaler J.S., Stout M.J., Karban R., Duffey S.S. (1996) Exogenous jasmonates simulate insect wounding in tomato plants (Lycopersicon esculentum) in the laboratory and field. J. Chem. Ecol. 22: 1767–1781. [DOI] [PubMed] [Google Scholar]
- Thaler J.S., Stout M.J., Karban R., Duffey S.S. (2001) Jasmonate-mediated induced plant resistance affects a community of herbivores. Ecol. Entomol. 26: 312–324. [Google Scholar]
- Tian D., Peiffer M., Shoemaker E., Tooker J., Haubruge E., Francis F. et al. (2012a) Salivary glucose oxidase from caterpillars mediates the induction of rapid and delayed-induced defenses in the tomato plant. PLoS One 7: e36168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian D., Tooker J., Peiffer M., Chung S.H., Felton G.W. (2012b) Role of trichomes in defense against herbivores: comparison of herbivore response to woolly and hairless trichome mutants in tomato (Solanum lycopersicum). Planta 236: 1053–1066. [DOI] [PubMed] [Google Scholar]
- Traw B.M., Dawson T.E. (2002) Differential induction of trichomes by three herbivores of black mustard. Oecologia 131: 526–532. [DOI] [PubMed] [Google Scholar]
- van der Fits L., Memelink J. (2000) ORCA3, a jasmonate-responsive transcriptional regulator of plant primary and secondary metabolism. Science 289: 295–297. [DOI] [PubMed] [Google Scholar]
- van Schie C.C., Haring M.A., Schuurink R.C. (2007) Tomato linalool synthase is induced in trichomes by jasmonic acid. Plant Mol. Biol. 64: 251–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdonk J.C., De Vos C.R., Verhoeven H.A., Haring M.A., van Tunen A.J., Schuurink R.C. (2003) Regulation of floral scent production in petunia revealed by targeted metabolomics. Phytochemistry 62: 997–1008. [DOI] [PubMed] [Google Scholar]
- Wagner G.J. (1991) Secreting glandular trichomes: more than just hairs. Plant Physiol. 96: 675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J., Yan L., Ren Q., Li C., Ge F., Kang L. (2013) Antagonism between herbivore-induced plant volatiles and trichomes affects tritrophic interactions. Plant Cell Environ. 36: 315–327. [DOI] [PubMed] [Google Scholar]
- Wu J., Baldwin I.T. (2009) Herbivory-induced signalling in plants: perception and action. Plant Cell Environ. 32: 1161–1174. [DOI] [PubMed] [Google Scholar]
- Yoshida Y., Sano R., Wada T., Takabayashi J., Okada K. (2009) Jasmonic acid control of GLABRA3 links inducible defense and trichome patterning in Arabidopsis. Development 136: 1039–1048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.