Abstract
Background
To determine the short-term effects following corticosteroid injection into the shoulder.
Methods
Type-specific shoulder injection was administered, followed by physiotherapy 7 days to 10 days later. One hundred and sixteen adult patients were prospectively followed-up. The primary outcome was a visual analogue score (VAS) for pain. Scores were recorded immediately before injection, 30 minutes after, daily until day 7 and then at 6 weeks. Post injection pain was defined as an increase of 2 or more points in the VAS score after the injection. The secondary outcome was determined at 6 months as successful discharge or progression onto surgery.
Results
The VAS showed a significant reduction from the pre-injection score for all patients at day 1 and was maintained until week 6. Forty-one (35.3%) patients experienced post-injection pain. The mean duration of symptoms was 3.9 days. At 6 months, 81 (69.8%) patients were discharged successfully and, at a mean of 23.2 months, did not require re-referral; 29 (25%) had surgery; and six (5.2%) were referred for a spinal opinion.
Conclusions
One in three patients developed delayed post-injection pain. Flare phenomenon had no determinate effect on outcome. Patients’ pain response by 6 weeks is predictive of final outcome at 6 months and may help clinicians plan further treatment without delay.
Keywords: corticosteroids injections, physiotherapy, post injection pain, shoulder injections, shoulder pain, surgery
Introduction
The prevalence of shoulder pain in adults is reported to be up to 34%,1 at any one time and annually, 1% to 2% will consult their general practitioners (GP) as a result.2 This accounts for approximately 2.4% of all GP consultations in the UK.3
Corticosteroid injection for shoulder pain is a well recognised4 and widely used cost effective treatment.5 National guidelines have recommended its use, as it is effective in reducing pain and improving function.6 Despite that the short-term effects are not quantified.7
The present study aimed to prospectively evaluate the short- and long-term benefits and risks of corticosteroid injections into the shoulder so that, in the future, we could inform our patients accurately about the likely outcome of this intervention.
Materials and methods
All adult patients referred with shoulder pain to the upper limb clinic between October 2012 and January 2015 were eligible for the study if an injection into the shoulder was part of their treatment plan. Exclusions included treatment for shoulder symptoms in the last 6 months; diagnoses of polymalgia rheumatica; symptoms secondary to acute shoulder trauma (e.g. dislocation, rotator cuff rupture); overt cervical radiculopathy; dementia; incapacitating psychiatric disorders; coexisting shoulder pathology (e.g. shoulder instability); patients on anticoagulants and those known to have thrombophilia predisposing to deranged coagulation; other contraindications as advised by the manufacturers.8
Non-operative treatment involved administration of a diagnosis-specific shoulder injection, followed by a referral to physiotherapy, which commenced after a 7-day to 10-day period. Prior to administering injections, all our patients were informed about all possible complications, including infection, and post injection pain. They were advised explicitly to seek urgent medical advice if their symptoms were suggestive of infection.
A non-touch injecting technique was used. The skin over the injection spot was cleaned using an alcohol swab, chloerhexadine or betadine.
The injection needle was changed between drawing the medications and injecting them into the shoulder. Local anaesthetics and corticosteroids were mixed in one syringe prior to administration. Injections into the subacromial bursa and glenohumeral joint were commenced at the posterior soft spot, using a 21-G needle, and directly as appropriate. A lateral approach was also used for in some patients for subacromial bursa. Those into the acromioclavicular (AC) joint were conducted via a direct anterosuperior approach, using a 23-G needle to account for the size of the joint gap. A simple fabric plaster was then applied to the injection site.
The injectate was either 40 or 80 mg of Depo-Medrone (Pfizer, Kent, UK) mixed with 5 ml of either 1% lignocaine, 2% lignocaine, 0.25% bupivicaine, or 0.5% bupivicane.
Patients completed a standardised visual analogue score (VAS) questionnaire for pain, recording their first score prior to the injection, the second 30 minutes after the injection and a further score recorded on each subsequent day for 6 days. A final score was recorded at their 6-week follow-up.
All of the eligible patients were then followed up to 6 months post-injection to assess their final outcome as either discharged or progressed to have surgery. All the patients’ records were assessed at the conclusion of the study for re-referral as a result of the recurrence of shoulder symptoms. This gave a recurrence-free follow-up period.
Post-injection pain or ‘flare reaction’ was defined as a two-point increase in the pre-injection VAS score at any time point on days 0 to 7. Severe pain reaction was considered as an increase in the pain score by 4 points or more.
The choice of 2 points as the definition of flare is based on the concept of minimal clinically important difference in pain, as has been assessed in multiple pain studies. The 2-point pain score has been found to be a meaningful change that is detectable and not related to measurement error.9–14
All statistical analyses were calculated using SPSS, version 20 (IBM Corp., Armonk, NY, USA.). p ≤ 0.05 was considered statistically significant. Fisher’s exact test and the t-test comprised the primary statistical tests used in the present study.
The study did not undergo institutional review board approval because it is considered as a service evaluation assessment.
Results
During the study period, 130 patients were eligible for inclusion. Fourteen patients were lost to follow-up and could not be contacted. Of the remaining 116 (89.2%) patients, 41 were men (35.3%) and 75 were women (64.7%). The mean age was 60.9 years (range 30 years to 88 years). The dominant shoulder-side was symptomatic in 64 patients (55%), whereas the nondominant was affected in 52 patients (45%).
The diagnoses were impingement in 64 patients (55.2%), rotator cuff tear in 20 (17.2%), frozen shoulder in 13 (11.2%), glenohumeral osteoarthritis in 11 (9.5%) and AC joint pain in eight (6.9%).
The injections were placed in the glenohumeral joint (for diagnoses of frozen shoulder and glenohumeral arthritis) in 24 patients (20.7%), in the AC joint in eight patients (6.9%) and in the sub-acromial bursa (impingement and rotator cuff tear) in 84 patients (72.4%).
A depot preparation of 40 mg of methylprednisolone (Depo-Medrone) was used in all patients, apart from those injected into the glenohumeral joint, where a double dose was used. Next, 5 ml of local anaesthetic was mixed with the methylprednisolone: 63 patients had 2% lignocaine, 28 patients had 0.5% bupivacaine, 20 patients had 1% lignocaine and five patients had 0.25% bupivacaine.
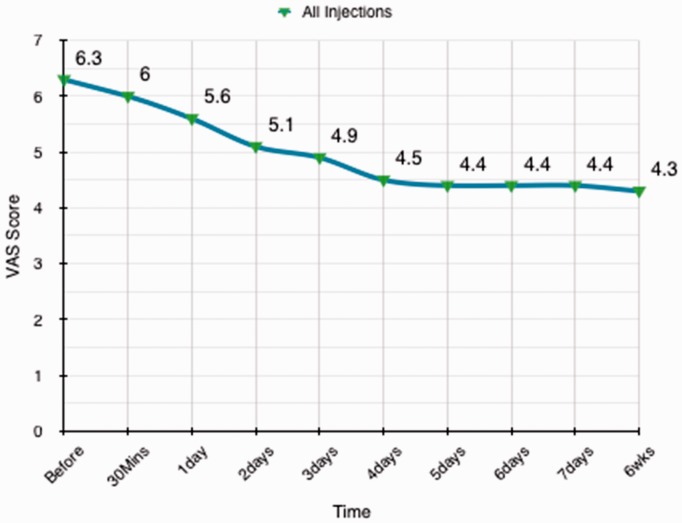
There was a statistical significant reduction of pain scores following injections from the pre-injection pain score levels in the total cohort of patients. This difference was not significant at 30 minutes after injection, although it was evident and statistically significant by day 1 and was maintained at week 1 and week 6 (p = 0.002; 95% confidence interval = 0.27 to 1.1, SE 0.215, SD 2.3) (Fig. 1)
Figure 1.
Mean visual analogue scale (VAS) pain scores of all patients are presented from baseline, before the injection, until the 6-week point.
A total of 41 patients (35.3%) experienced post-injection pain/flare. The mean duration of symptoms was 3.88 days (range 1 day to 7 days). Nine patients (7.7%) experienced severe flare (4-point increase in pain score).
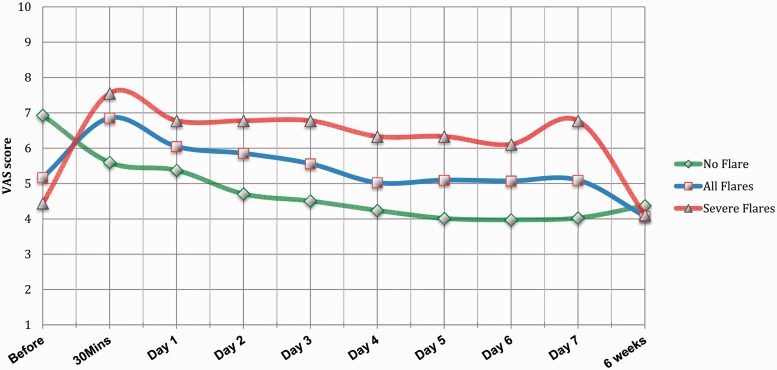
A subgroup of 24 patients from those who suffered post-injection pain/flare experienced the pain within the first 30 minutes. Out of these 24 patients, 11 patients (45.83%) continued to be symptomatic for a mean of 3.8 days (range 1 day to 7 days), whereas 13 patients (54.16%) had symptoms only for the day of the injection (Fig. 2)
Figure 2.
Mean visual analogue scale (VAS) pain scores for patients who developed post injection severe pain/flare (red, triangle), all patient who developed post injection pain/flare (blue, square) and patients who did not develop this phenomena (green, diamond).
Reviewing the electronic medical records of the total cohort who had injections, none had presented with symptoms suggestive of infection following their injections. At the 6-week follow-up point, none reported any concerns about infections or had consulted their G.P.
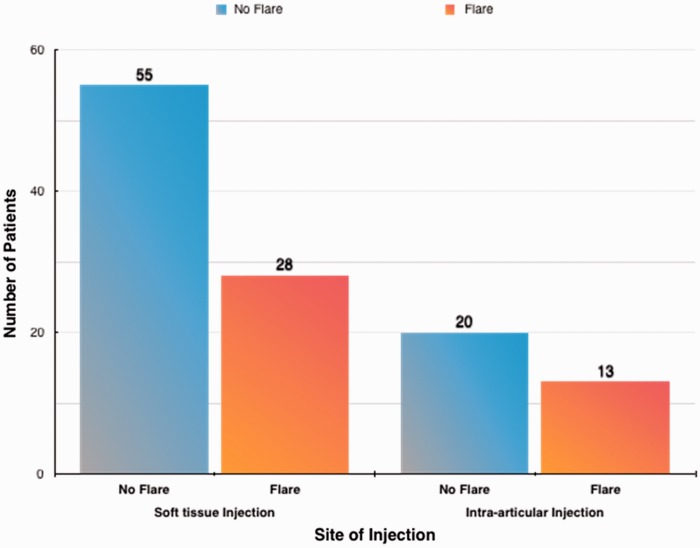
Gender and handedness were not statistically correlated with development of the flare reaction (p > 0.05). The flare reaction was not dependent on the location of the injection (p = 0.67) (Fig. 3).
Figure 3.
Graph describing the number of patients who had soft tissue injections and those who had intra-articular injections. Dark orange coloured columns represent those who did not develop post injection pain/flare. The light blue columns represent those who developed post injection pain/flare.
At the final follow-up, there was no clinical evidence of hypopigmentation, subcutaneous fat necrosis, atrophy or tendon rupture. None of the patients developed soft tissue infection or septic arthritis.
At 6 months post-injection, 81 (69.8%) patients were discharged successfully, were satisfied by their current level of symptoms, and were in no need of further intervention at the time. They were all followed up in accordance with our methodology, and the mean follow-up for recurrence-free re-referral period was 23.2 months (range 6 months to 32 months). One patient died, at 15 months post-injection, of unrelated causes.
Twenty-nine (25%) patients had surgery or listed for surgery: 16 patients with primary diagnoses of impingement, two patients with AC pain, two patients with frozen shoulders, four patients with glenohumeral osteoarthritis and five patients with rotator cuff tears.
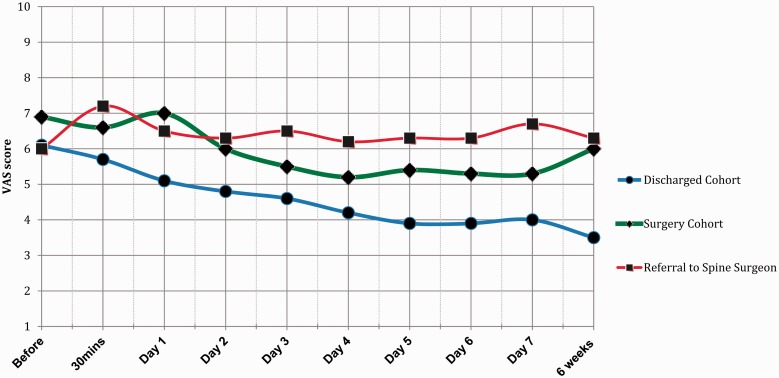
Six (5.2%) patients with concomitant neck pain, in whom it was unclear where the predominant source of symptoms was, showed no improved response to shoulder injection (Fig. 4). This was considered to represent a ‘diagnostic’ indication that neck pain predominated and, as a result, they were referred to the spinal surgeons.
Figure 4.
Mean visual analogue scale (VAS) scores for patients who were managed conservatively and discharged successfully by 6 months (blue, circle), patients who were listed for/ had surgery by 6 months for on-going symptoms (green, diamond) and patients whose symptoms became overtly suggestive of cervical spinal pathology (red, square).
Those patients who were successfully discharged had a mean pre-injection pain VAS score of 6.1 (range 1 to 10), which improved after 6 weeks to a mean of 3.5 (range 0 to 9). On the other hand, the cohort that proceeded to have surgery had a mean VAS score of 6.9 (range 2 to 10) before the injection. This only showed an improved mean VAS score of 6 (range 1 to 10) at 6 weeks post-injection with physiotherapy. There was a statistical significance between both groups (p ≤ 0.0001) at 6 weeks (Fig. 4).
Discussion
Subsequent to the first two reported studies in the 1950s conducted by Hollander et al.15 and Wrenn et al.16, on the effects and benefits of corticosteroids in management of intra-articular and soft tissues conditions respectively, their use in injectable forms has gained universal acceptance.17
The majority of shoulder surgeons use corticosteroid injections as an integral part of their clinical management for patients presenting to them with shoulder pain,18 and recent national commissioning guidelines for subacromial impingement and rotator cuff tendonopathy recommend their use in primary care.6
In this prospective study of adult patients with shoulder pain treated with corticosteroid injections and physiotherapy, we observed a statistically significant improvement in their reported pain scores. Although this was not noticeable in the first 30 minutes after the injection, it was noted daily from day 1 until day 7, and at 6 weeks post-injection. The percentages of shoulder pathologies in the present study are in concordance with previously reported incidences and prevalence.19,20
There is no standard reported technique for shoulder injection; however, using our approach, our results support the safety of the simple non-touch technique. This demonstrates as well that the technique is effective in producing the desirable effect in line with the recent Cochrane review suggesting that there is no added benefit from image guided injections.21
Other studies have reported a wide range of incidences of flare reaction 1% to 81%15,22 secondary to corticosteroid injection, at different anatomical locations. Gaujoux-Viala et al.23 conducted a meta-analysis on randomized controlled trials (RCT) assessing the efficacy and safety of steroid injections for shoulder and elbow tendonitis. The incidence of flare in elbow injections was quoted to be between 10.7% and 11%,24,25 whereas there was no reported incidence for the shoulder. Der Van Windet et al.23 conducted an RCT assessing the efficacy of corticosteroid injections versus physiotherapy for treatment of painful stiff shoulder in general practices settings. They reported a collective incidence of increasing pain after treatment in both groups, lasting between less than 1 day to more than 2 days with respect to 44% in the cohort who were treated with injections and 52.6% in those who had physiotherapy only.26 These authors did not perform a detailed assessment regarding the incidence or characteristics of post injection pain.
There are no previous methodological reports on post corticosteroid shoulder injection pain in the literature, and all the quoted figures are anecdotal. Thus we report, to our knowledge, the first clinical study of the incidence of delayed pain (flare reaction) in shoulder injections to be 35.3%, with neither gender, nor handedness predicting the development of this reaction.
Hollander et al.15 published his experience in over 100,000 patients treated with intra-articular injections, reporting a post injection inflammatory flare in approximately 1% to 2% of the patients. It was reported to be related to larger doses and traumatic injections. McCarty and Hogan27 in 1964 were the first to study this phenomenon in detail. They reported that the most likely explanation is that the steroid crystal itself acts as an irritant, whereas the steroid moiety in solution exerts an anti-inflammatory effect.
By contrast to a previous review reporting that the flare reaction usually began several hours following injection, and subsided spontaneously within 72 hours,28 we observed that 58.5% of those patients who developed post injection flare, developed it within 30 minutes of the injection. Those patients categorically confirmed that this pain was not related to the injection-prick itself. Approximately half of this group was symptomatic for only the day of the injection, and the other half went on to be symptomatic for a mean of 3.8 days.
MacMahon et al.28 reported that severe flare may be difficult to distinguish from sepsis, this was supported by Ostergaard and Halberg29 who recommend joint aspiration if the flare lasted longer than 24 hours.
In the present study, none of the 130 patients developed infection subsequent to the injection, Nevertheless, a high index of suspicion and judgment on an individual case basis is our recommended approach towards possible post-injection septic arthritis, bearing in mind the estimated overall incidence of steroid injection related septic arthritis is 1 in 14 000 to 1 in 50 000.30,31
We also found no evidence of hypopigmentation, subcutaneous fat necrosis, atrophy, or tendon rupture in our patients’ cohort.
Studies conducted in the 1960s by Hollander et al.32 and Kendal33 support the idea that development of a flare reaction neither predicts good, nor poor outcome. We also found no difference in outcome between those that developed a flare and those that did not. We note, from our results, that patients who developed flare had an initial lower VAS score (mean of 5.17) in comparison to patients who did not develop flare (VAS mean of 6.9). We have no explanation for this observation.
Our results have demonstrated that those patients who made a significant improvement of VAS score at 6 weeks ultimately did not require surgical intervention and were successfully discharged by 6 months. Apart from those patients with concomitant cervical spinal pathology, all other patients who showed no significant improvement in VAS score at 6 weeks went on to surgical treatment. Cummins et al.34 described a similar response by patients with purely impingement symptoms.34
These findings may help clinicians when treating patients with shoulder pain; should there be an inadequate response at 6 weeks to injection, delaying further treatment is unnecessary because a belated positive response is unlikely.
The use of different types of local anaesthetic preparations could be perceived as a limitation to the present study, although our aim was to represent the different licensed preparations available for use in different clinical settings. Both preparations groups have comparable bioavailability characteristics. Lidocaine has an average half-life of 1.5 hours to 2 hours, with an estimated duration of action of 0.5 hours to 3 hours.35 The bupivacaine has an average half-life of 2.7 hours, and an estimated duration of action of 2 hours to 8 hours.36
Conclusions
In summary, in a pragmatic prospectively observed adult cohort with shoulder pain, who were managed conservatively with steroid injections and physiotherapy, steroid injections helped to improve the pain in a significant and sustained manner up until 6 weeks. One in three patients developed post injection flare, and the mean duration for symptoms was just under 4 days. Developing a flare reaction was associated with neither a good, nor a bad response to therapy. None of the injections were complicated by infection. This management approach resulted in 70% of patients being successfully discharged, with a mean period of no further re-referral of approximately 2 years.
Acknowledgements
The authors would like to thank Ms Lorraine Timmons and Ms Priscilla MacGregor for their expert secretarial support.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The preliminary results were presented at the 24th BESS scientific meeting in June 2013. The final results were presented at the BOA annual congress in September 2016.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Review and Patient Consent
The study did not undergo institutional review board approval because it is considered as a service evaluation assessment.
References
- 1.Pope DP, Croft PR, Prttchard CM, Macfarlane GJ, Selman AJ. The frequency of restricted range of movement in individuals with self-reported shoulder pain: results from a population-based survey. Rheumatology 1996; 35: 1137–1141. [DOI] [PubMed] [Google Scholar]
- 2.Urwin M, Symmons D, Allison T, et al. Estimating the burden of musculoskeletal disorders in the community: the comparative prevalence of symptoms at different anatomical sites, and the relation to social deprivation. Ann Rheum Dis 1998; 57: 649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linsell L, Dawson J, Zondervan K, et al. Prevalence and incidence of adults consulting for shoulder conditions in UK primary care; patterns of diagnosis and referral. Rheumatology 2006; 45: 215–221. [DOI] [PubMed] [Google Scholar]
- 4.Gruson KI, Ruchelsman DE, Zuckerman JD. Subacromial corticosteroid injections. J Shoulder Elbow Surg 2008; 17: S118–S130. [DOI] [PubMed] [Google Scholar]
- 5.James M, Stokes EA, Thomas E, Dziedzic K, Hay EM. A cost consequences analysis of local corticosteroid injection and physiotherapy for the treatment of new episodes of unilateral shoulder pain in primary care. Rheumatology 2005; 44: 1447–1451. [DOI] [PubMed] [Google Scholar]
- 6.Kulkarni R, Gibson J, Brownson P, et al. Subacromial shoulder pain. Shoulder Elbow 2015; 7: 135–143. [DOI] [PMC free article] [PubMed]
- 7.Murphy RJ, Carr AJ. Shoulder pain. Clin Evid (Online) 2010; 2010: 1107–1107. [PMC free article] [PubMed] [Google Scholar]
- 8.Author: Depo-Medrol (methylprednisolone acetate injectable [cited 2016Nov15]. http://www.pfizerinjectables.com/sites/default/files/factsheet-pdf/00009-0280-03_20140429.pdf (accessed 15 November 2016).
- 9.Childs JD, Piva SR, Fritz JM. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine 2005; 30: 1331–1334. [DOI] [PubMed] [Google Scholar]
- 10.Goldfarb CA, Gelberman RH, McKeon K, Chia B, Boyer MI. Extra-articular steroid injection: early patient response and the incidence of flare reaction. J Hand Surg 2007; 32: 1513–1520. [DOI] [PubMed] [Google Scholar]
- 11.Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL. Defining the clinically important difference in pain outcome measures. Pain 2000; 88: 287–294. [DOI] [PubMed] [Google Scholar]
- 12.Salaffi F, Stancati A, Silvestri CA, Ciapetti A, Grassi W. Minimal clinically important changes in chronic musculoskeletal pain intensity measured on a numerical rating scale. Europ J Pain 2004; 8: 283–291. [DOI] [PubMed] [Google Scholar]
- 13.Hollander JL, Brown EM, Jr., Jessar RA, Brown CY. Hydrocortisone and cortisone injected into arthritic joints: comparative effects of and use of hydrocortisone as a anti arthritic agent. J Am Med Assoc 1951; 147: 1629–1635. [DOI] [PubMed] [Google Scholar]
- 14.Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001; 94: 149–158. [DOI] [PubMed] [Google Scholar]
- 15.Hollander JL. Intrasynovial corticosteroid therapy in arthritis. Maryland State Med J 1970; 19: 62–66. [PubMed] [Google Scholar]
- 16.Wrenn RN, Goldner JL, Markee JL. An experimental study of the effect of cortisone on the healing process and tensile strength of tendons. J Bone Joint Surg Am 1954; 36: 588–601. [PubMed] [Google Scholar]
- 17.Coombes BK, Vicenzino B. Pragmatic study of corticosteroid injections and manual physical therapy for the shoulder impingement syndrome. Ann Int Med 2014; 161: 224–225. [DOI] [PubMed] [Google Scholar]
- 18.Bryceland JK, Drury C, Tait GR. Current UK practices in the management of subacromial impingement. Shoulder Elbow 2015; 7: 164–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chard MD, Hazleman R, Hazleman BL, King RH, Reiss BB. Shoulder disorders in the elderly: a community survey. Arthritis Rheum 1991; 34: 766–769. [DOI] [PubMed] [Google Scholar]
- 20.Vecchio P, Kavanagh R, Hazleman BL, King RH. Shoulder pain in a community-based rheumatology clinic. Rheumatology 1995; 34: 440–442. [DOI] [PubMed] [Google Scholar]
- 21.Bloom JE, Rischin A, Johnston RV, Buchbinder R. Image-guided versus blind glucocorticoid injection for shoulder pain. Cochrane Database Syst Rev 2012; 8: CD009147–CD009147. [DOI] [PubMed] [Google Scholar]
- 22.Brinks A, Koes BW, Volkers AC, Verhaar JA, Bierma-Zeinstra SM. Adverse effects of extra-articular corticosteroid injections: a systematic review. BMC Musculoskelet Disord 2010; 11: 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaujoux-Viala C, Dougados M, Gossec L. Efficacy and safety of steroid injections for shoulder and elbow tendonitis: a meta-analysis of randomised controlled trials. Ann Rheum Dis 2009; 68: 1843–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haker E, Lundeberg T. Elbow-band, splintage and steroids in lateral epicondylalgia (tennis elbow). Pain Clinic 1993; 6: 103–112. [Google Scholar]
- 25.Price R, Sinclair H, Heinrich I, Gibson T. Local injection treatment of tennis elbow – hydrocortisone, triamcinolone and lignocaine compared. Rheumatology 1991; 30: 39–44. [DOI] [PubMed] [Google Scholar]
- 26.der van Windt DA, Koes BW, Deville WL, Boeke AJ, De Jong BA, Bouter LM. Effectiveness of corticosteroid injections versus physiotherapy for treatment of painful stiff shoulder in primary care: randomised trial. BMJ 1998; 317: 1292–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarty DJ, Hogan JM. Inflammatory reaction after intrasynovial injection of microcrystalline adrenocorticosteroid esters. Arthritis Rheum 1964; 7: 359–367. [DOI] [PubMed] [Google Scholar]
- 28.MacMahon PJ, Eustace SJ, Kavanagh EC. Injectable corticosteroid and local anesthetic preparations: a review for radiologists 1. Radiology 2009; 252: 647–661. [DOI] [PubMed] [Google Scholar]
- 29.Østergaard M, Halberg P. Intra-articular corticosteroids in arthritic disease. BioDrugs 1998; 9: 95–103. [DOI] [PubMed] [Google Scholar]
- 30.Gray RG, Gottlieb NL. Intra-articular corticosteroids an updated assessment. Clin Orth Rel Res 1983; 177: 235–263. [PubMed] [Google Scholar]
- 31.Hollander JL. The use of intra-articular hydrocortisone, its analogues, and its higher esters inarthritis. Ann N Y Acad Sci 1955; 61: 511–516. [DOI] [PubMed] [Google Scholar]
- 32.Hollander JL, Jessar RA, Brown EM., Jr Intra-synovial corticosteroid therapy: a decade of use. Bull Rheum Dis 1961; 11: 239–239. [PubMed] [Google Scholar]
- 33.Kendall PH. Local corticosteroid injection therapy – III. Rheumatology 1963; 7: 31–38. [PubMed] [Google Scholar]
- 34.Cummins CA, Sasso LM, Nicholson D. Impingement syndrome: temporal outcomes of non-operative treatment. J Shoulder Elbow Surg 2009; 18: 172–177. [DOI] [PubMed] [Google Scholar]
- 35.American Society of Health-System Pharmacists. http://www.drugs.com/monograph/lidocaine-hydrochloride-local.html (accessed March 2016).
- 36.American Society of Health-System Pharmacists. http://www.drugs.com/monograph/bupivacaine-hydrochloride.html (accessed March 2016).