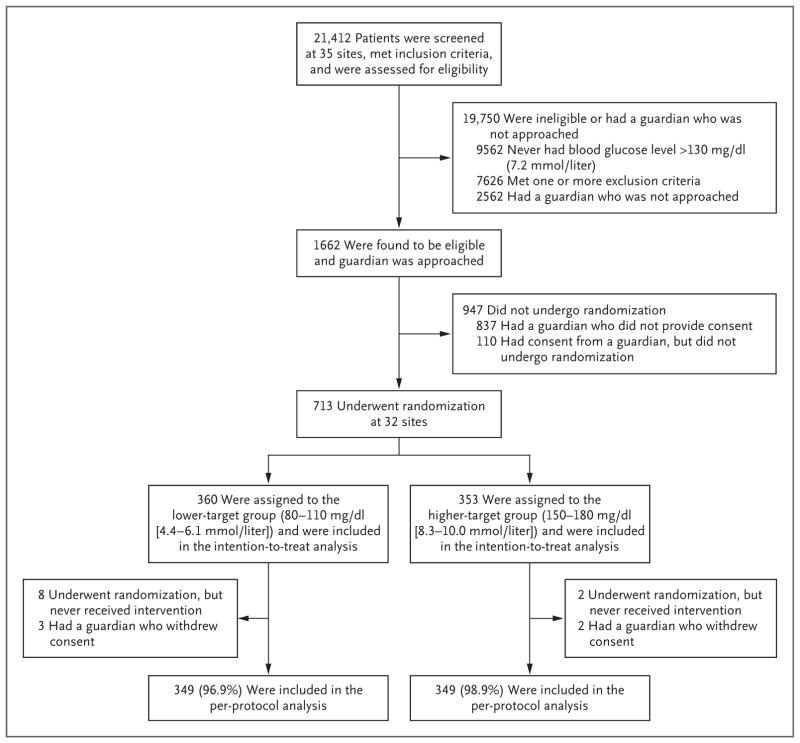
Figure 1. Assessment, Randomization, and Follow-up of the Study Patients.
The informed-consent rate was 50% (825 of 1662 patients). Only patients with a measured blood glucose level greater than 130 mg per deciliter were assessed for exclusion criteria. Two additional patients underwent randomization and were in the study when it was stopped early; these patients are not included in the analyses according to the stipulation of the data and safety monitoring board. Additional details are provided in Tables S1 and S2 in the Supplementary Appendix.