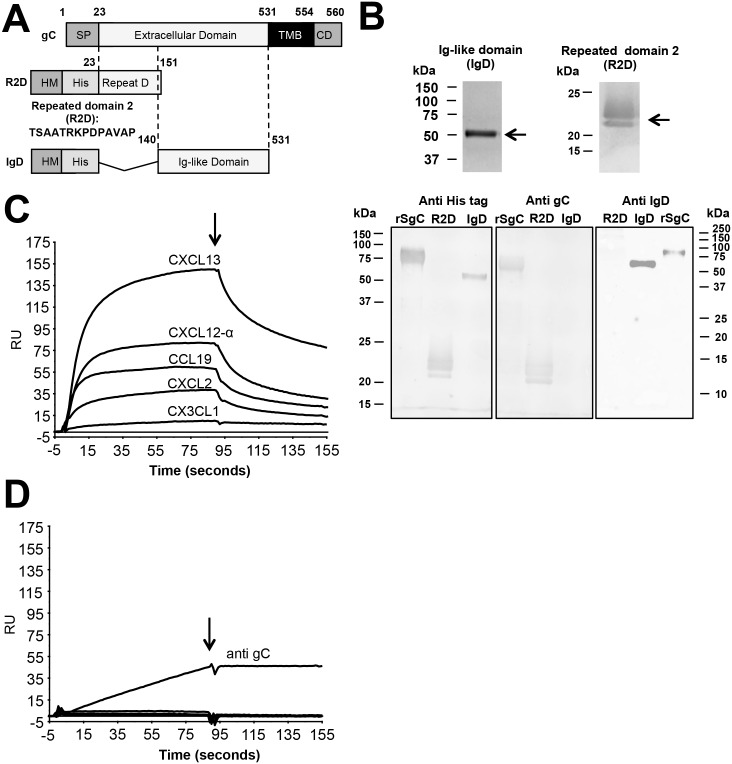
Fig 4. Identification of the rSgC binding domain responsible for interaction with chemokines.
(A) Schematic representation of full-length gC protein (top construct) and deletion constructs containing either amino acids 23–151 (R2D, middle construct) or amino acids 140–531 (IgD, bottom construct). The numbers indicate amino acid positions within VZV gC Dumas strain. To improve secretion in insect cells the VZV gC signal peptide (SP) was substituted by that of the honey bee melittin (HM). The introduction of the N-terminal histidine tag (His) allowed purification of the proteins by affinity chromatography. (B) Purified proteins were detected by Coomassie staining (upper panels) or by Western blotting (bottom panels) using antibodies: anti His-tag (left panel), anti R2D (middle panel) and anti IgD (right panel). Left and middle blots were obtained following transfer from the same gel, whereas the right blot comes from an independent gel. (C,D) Sensorgrams showing the association and dissociation phases of the interaction between chemokines (CXCL2, CXCL12-α, CXCL13, CCL19 and the negative control CX3CL1 at 100 nM) and IgD (C) or R2D (D). The same chemokines were injected in the IgD and R2D chips. The arrow indicates the end of the chemokine injection. (D) An antibody targeted to gC was injected into the R2D chip at a concentration of 10 ng/μl. Abbreviations: RU, resonance units; kDa, kiloDaltons; TMB, transmembrane; CD, cytoplasmic domain.