Figure 4.
Truncated BP80 Lacking the Ligand Binding Domain Interferes with Vacuolar Sorting.
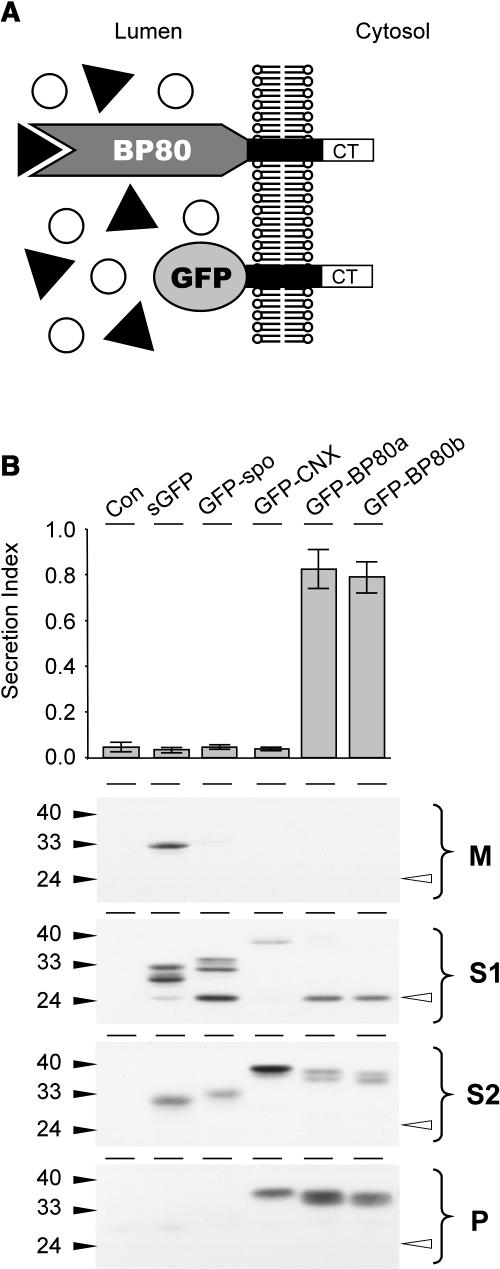
(A) Schematic illustration of the nature and topology of the truncated BP80 fusions in relation to the full-length BP80. The complete ligand binding domain of the Arabidopsis BP80 isoforms a, b, d, and f (Hadlington and Denecke, 2000) were replaced by GFP, which results in the truncated molecules (GFP-BP80a, GFP-BP80b, GFP-BP80d, and GFP-BP80f) that do not interact with BP80-ligands but yet display the same polypeptide on the cytosolic face of the membrane as the wild-type BP80 counterparts.
(B) Protoplasts were transfected with plasmid-encoding amy-spo alone or together with 20 μg of plasmid encoding either sGFP, sGFP-spo, GFP-calnexin (GFP-CNX), or GFP-BP80 fusions (represented here by GFP-BP80a and b) and incubated for 24 h, after which medium and cells were harvested. The top panel shows the secretion index of cotransfected amy-spo; annotations are as in the previous figures. Notice that only GFP-BP80a and GFP-BP80b induce the secretion of amy-spo, whereas sGFP, GFP-spo, and GFP-CNX have no effect. Cells were fractionated to obtain extracts enriched in soluble protein released by osmotic shock (S1), proteins released after sonicating the first pellet (S2), and membrane spanning proteins (P). These cell fractions together with the medium (M) were analyzed (bottom panel) to test the intracellular partitioning of the various GFP fusions. Molecular mass markers are indicated in kD. Notice the lower molecular mass degradation fragment, the GFP core (white arrowhead) that is detected exclusively in S1 for all GFP fusions except for GFP-CNX.