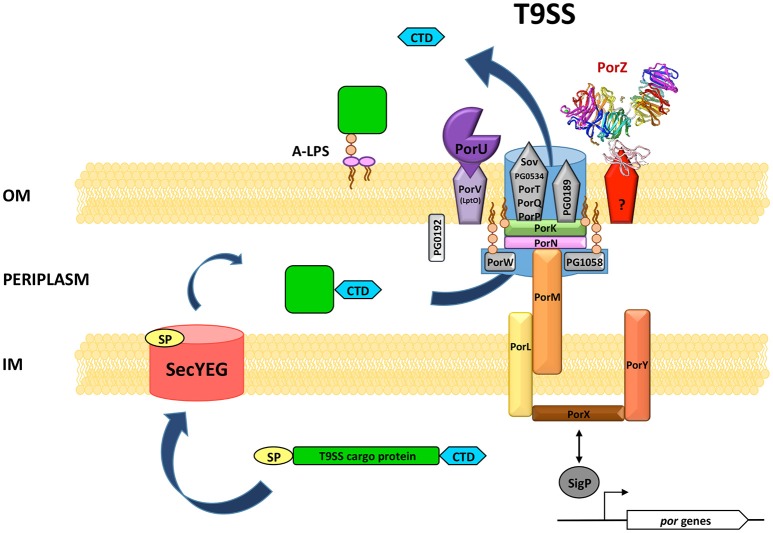
Figure 3.
Hypothetical model of the structure and function of P. gingivalis T9SS. The overall translocon structure and the protein(s) forming a pore in the OM (outer membrane) have not yet been characterized. Therefore, it is shown as a background blue shape accommodating known components. Interacting proteins are situated in close proximity. OM β-barrel proteins are depicted as pentagons. PorZ is presently the only T9SS protein with the known atomic structure. The mode of its association with the translocon is not yet defined. PorK, PorW, and PG1058 are lipoproteins anchored into the inner surface of the OM. PG0192 protein precise localization and possible interactions are not known. A T9SS cargo protein is equipped with two sorting signals: N-terminal signal peptide (SP) directing the protein to the general secretion system SecYEG and conserved C-terminal domain (CTD) recognized by T9SS. After translocation through the IM (inner membrane) most proteins acquire their proper fold in the periplasm. Next, CTD directs the protein for further translocation across the OM through T9SS. Finally, CTD is cleaved off by PorU sortase and a secreted protein is modified by attachment of A-LPS resulting in the anchorage of cargo protein to the cell surface. Two component system PorX/PorY and sigma factor SigP have regulatory effect on por genes. Although, they are not physical elements of T9SS, PorX was shown in vitro to interact with PorL.