Fig. 6.
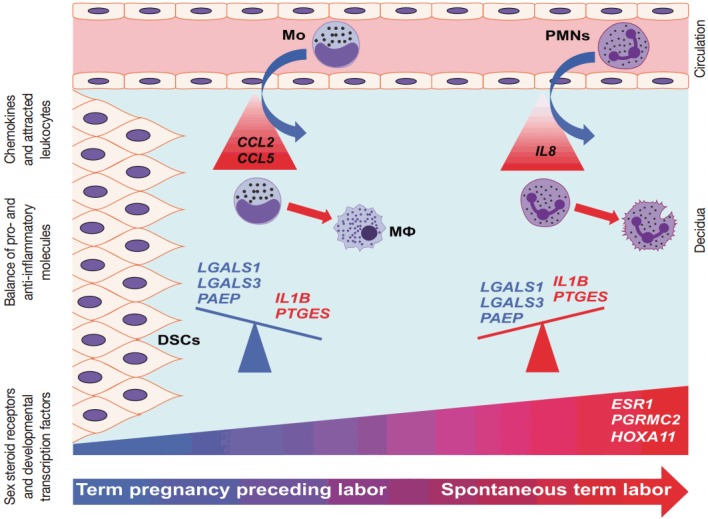
Conceptual framework. The increased decidual expression of a signaling factor responsible for decidual maturation and development (HOXA11) is preceded by the increased expression of chemokines (CCL2 and CCL5), which may stimulate the early recruitment of monocytes into the decidua as the onset of labor approaches. These immune cells will be activated by the local microenvironment and contribute to the orchestration of inflammation. With the initiation of parturition, the decidual expression of anti-inflammatory mediators (LGALS1, LGALS3, and PAEP) decreases, while the expression of pro-inflammatory mediators (IL1B and PTGES) and steroid receptors (ESR1 and PGRMC2) increases, contributing to “functional progesterone withdrawal” and heightened inflammation, eventually leading to uterine contractions, cervical ripening, and membrane rupture. The relatively late increase of IL8 expression will be followed by the recruitment of neutrophils, which plays a key role in tissue repair. These results strengthen earlier findings on the decidua being the earliest among gestational tissues that get primed during parturition. CCL2, chemokine C-C motif ligand 2; CCL5, chemokine C-C motif ligand 5; DSCs, decidual stromal cells; ESR1, estrogen receptor 1; HOXA11, homeobox A11; IL1B, interleukin-1β; IL8, interleukin 8; LGALS1, galectin-1; LGALS3, galectin-3; Mo, monocytes; MΦ, macrophages; PAEP, progestogen-associated endometrial protein; PGRMC2, progesterone receptor membrane component 2; PMNs, neutrophil granulocytes; PTGES, prostaglandin E synthase.
