Fig. 8.
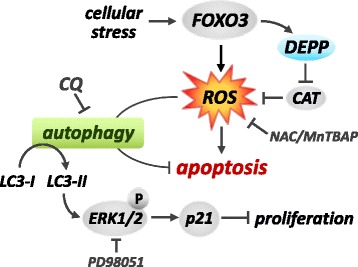
Proposed model for the FOXO3-DEPP-ROS axis in the regulation of life - death balance via autophagy. Cellular stress activates the transcription factor FOXO3 which in turn triggers ROS accumulation and DEPP expression. DEPP represses the cellular ROS detoxification capacity by reducing the CAT/catalase activity and thereby further increases ROS accumulation, which induces both, autophagy and apoptosis. Hence, FOXO3/DEPP modulate an autophagy program via increased cellular ROS to efficiently degrade damaged proteins and organelles and thereby protect neuroblastoma cells from apoptosis. The ROS scavenger NAC and MnTBAP efficiently repress FOXO3/DEPP-triggered autophagy. Repression of DEPP results in reduced ROS levels [9] and consequently neither apoptosis nor autophagy are executed. Inhibition of FOXO3/DEPP-mediated autophagy via CQ or LC3-specific siRNA sensitizes neuroblastoma cells to chemotherapy-induced, apoptotic cell death. DEPP-triggered ROS mediate LC3-lipidation, ERK1/2 phosphorylation, and consequently p21 induction that results in reduced cellular proliferation. Both, LC3 - knockdown and ROS - inhibition by NAC, attenuate DEPP-triggered ERK1/2 phosphorylation. Inhibition of ERK1/2 phosphorylation by PD98051 attenuates DEPP-mediated p21 induction and thereby restores proliferation
