Fig. 5.
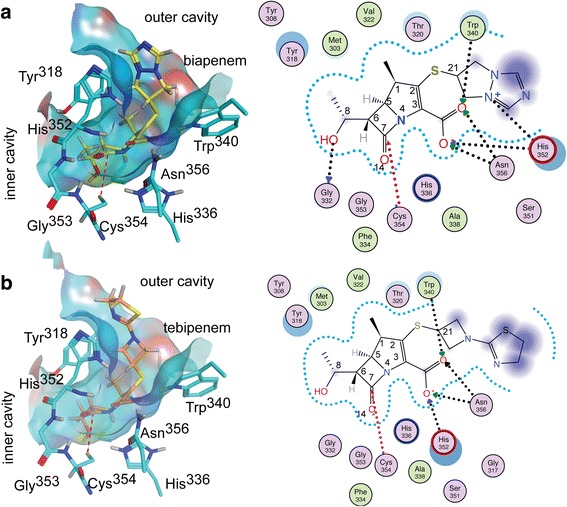
Predicted binding models of the biapenem and tebipenem interactions with LdtMt2. Docking results of the binding of intact (a) biapenem and (b) tebipenem to the outer cavity of LdtMt2. Left panels of (a) and (b) show the solvent accessible surface corresponding to the outer cavity and tunnel connecting to the inner cavity. The carbapenems (biapenem carbon atoms are yellow; tebipenem carbon atoms are orange) and LdtMt2 residues that participate in binding (cyan). The protein-ligand interactions are shown in the right panels. Residues circled are in Van der Waals contact with the ligand; those colored green and pink are hydrophobic and hydrophilic residues, respectively. Hydrogen bonds are marked as black dashed arrows starting in the proton donor. The red dashed arrow highlights the Cys354-C7 tether used for the steered docking simulation. Purple clouds around carbapenem atoms indicate solvent exposure, and the size of the clouds indicates the degree of exposure. Offset blue circles indicate partial exposure of the protein residue. The drawing and analysis were performed using MOE
