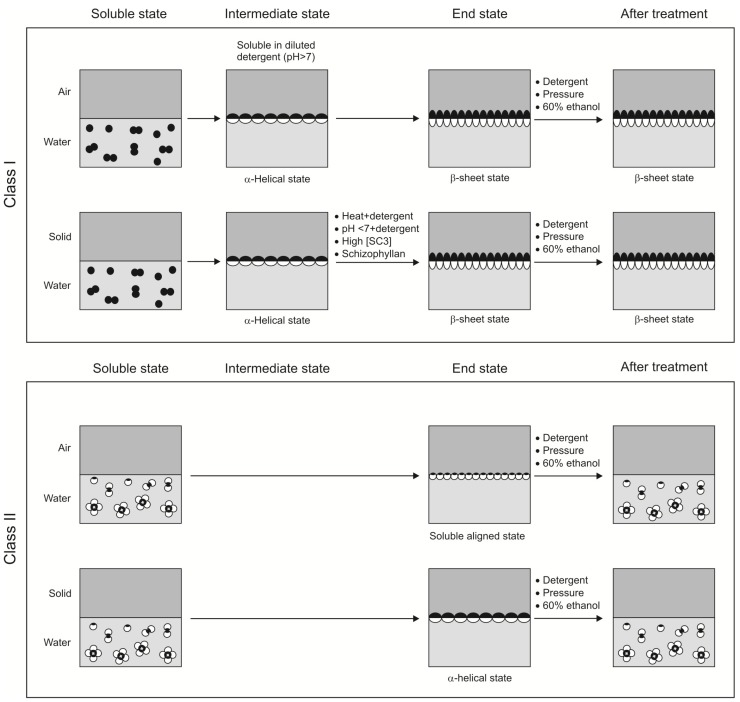
Figure 2.
Model for assembly of class I and II hydrophobins at a hydrophilic-hydrophobic interface. At a water-air interface, class I hydrophobins (e.g., SC3; upper panel) spontaneously self-assemble via an α-helical intermediate state into a stable β-sheet end configuration. In contrast, upon contact with hydrophobic solids (e.g., Teflon) in water, SC3 is arrested in the intermediate α-helical configuration. The transition to the stable β-sheet end form is promoted by high protein concentration, presence of the polysaccharide schizophyllan (SPG) and the combination of heat or low pH and detergents. Class II hydrophobins (lower panel) do not assemble via an intermediate form. At the water-air interface, the conformation remains the same compared to the soluble state. The molecules orient themselves at the interface with the hydrophobic patch directed towards the air and the hydrophilic part directed to the water (soluble aligned state). On a solid-water interface, a conformational change into an α-helical form is observed. The end state of class I hydrophobins (upper panel) is very stable and cannot be dissociated by pressure, detergent or 60% ethanol. In contrast, the end form of class II hydrophobins (lower panel) readily dissolves under these conditions.