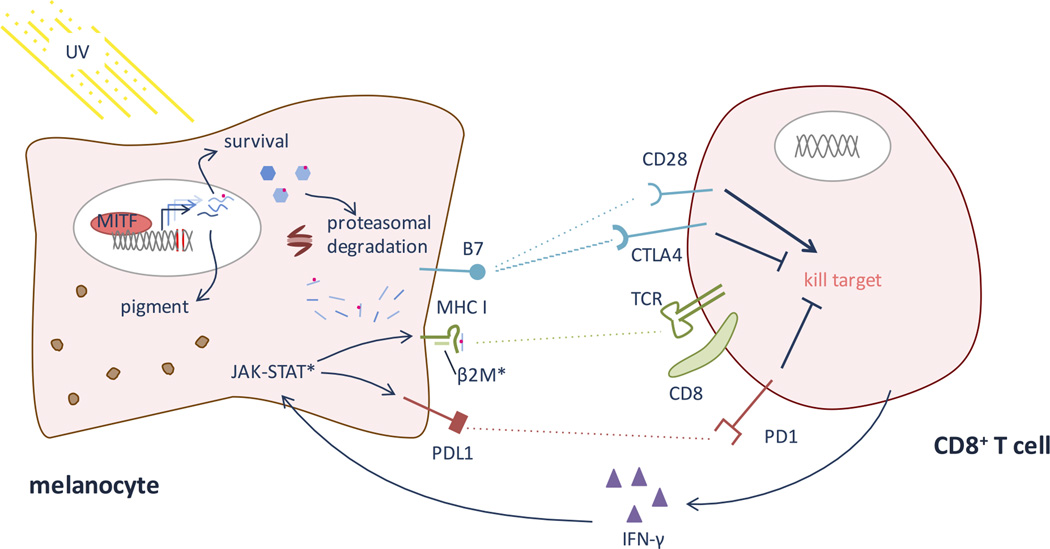
Figure 2. T cell recognition of melanocyte antigens and neo-epitopes.
When MITF is induced (see Figure 1), it causes up-regulation of many genes relevant for pigmentation and cell survival. Because of accumulated UV-induced DNA damage, some of these genes likely harbor mutations. The proteasome system degrades proteins in the cell, allowing presentation of endogenous antigens on MHC class I. Thus, MHC will present some neo-epitopes. In addition to melanocyte-intrinsic survival factors, the melanocyte must survive CD8 T cell recognition of these neo-epitopes, via TCR binding to peptide-MHC, and a cytotoxic response. Co-stimulatory molecules, notably the B7-CD28/CTLA4 and PDL1-PD1 pathways, help dictate whether the CD8 T cell will respond by killing the melanocyte or becoming tolerant upon TCR engagement of a foreign epitope. PD1 and CTLA4 send suppressive, tolerizing signals, while CD28 engagement signals CD8 activation of a cytotoxic response. Checkpoint inhibitors – notably anti-PD1, anti-PDL1, and anti-CTLA antibodies – encourage the CD8 T cell to kill a target cell when its TCR engages a peptide-MHC complex perceived as foreign. Once an immunogenic epitope is recognized, epitope spreading can occur, leading to activation against other antigens co-expressed on the melanocyte. (*) Represent main sites of known resistance to immunotherapy. Melanomas can evolve to evade immune recognition of their neo-epitopes by down-regulating MHC I, often via mutations in β2 microglobulin (β2M). Additionally, mutations in the JAK-STAT pathway prevent IFN-γ mediated up-regulation of PDL1 and MHC class I.