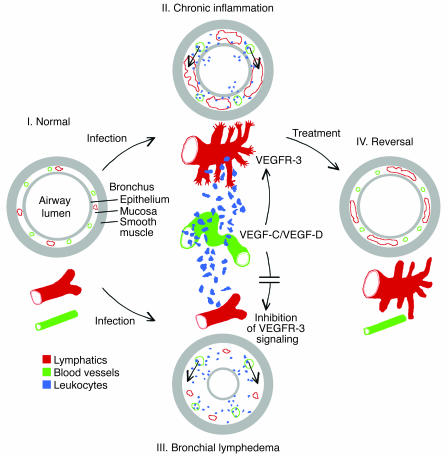
Figure 7.
Role of lymphangiogenesis in airway inflammation. The diagram shows relationships of changes in lymphatic vessels (red), blood vessels (green), inflammatory cells (blue), and plasma leakage (short arrows) in inflamed airway mucosa. Cross-sections of bronchi and vessel schematics compare 4 conditions. (I) In normal airways, blood vessels have little or no leukocyte traffic; baseline leakage drains via lymphatic vessels. (II) After infection, activated antigen-presenting cells traffic from airways to local lymph nodes, evoking an immune response. Mucosal capillaries remodel into activated venules that mediate leukocyte influx. These cells release VEGF-C and VEGF-D, which drive lymphangiogenesis via VEGFR-3 signaling in lymphatic endothelial cells. The lymphatic network expands by sprouting from existing lymphatic vessels to accommodate the increased leakage from venules and increased trafficking of immune cells to lymph nodes. (III) If lymphangiogenesis does not occur, leakage exceeds drainage, bronchial lymphedema develops, trafficking of immune cells to lymph nodes decreases, and the immune response is reduced. (IV) Treatment decreases the inflammatory stimulus, allowing blood vessels to return to the baseline state. Leukocyte influx decreases, and the stimulus for lymphangiogenesis diminishes, but lymphatic vessels that formed during the infection persist – ready for the next infection.