Figure 7.
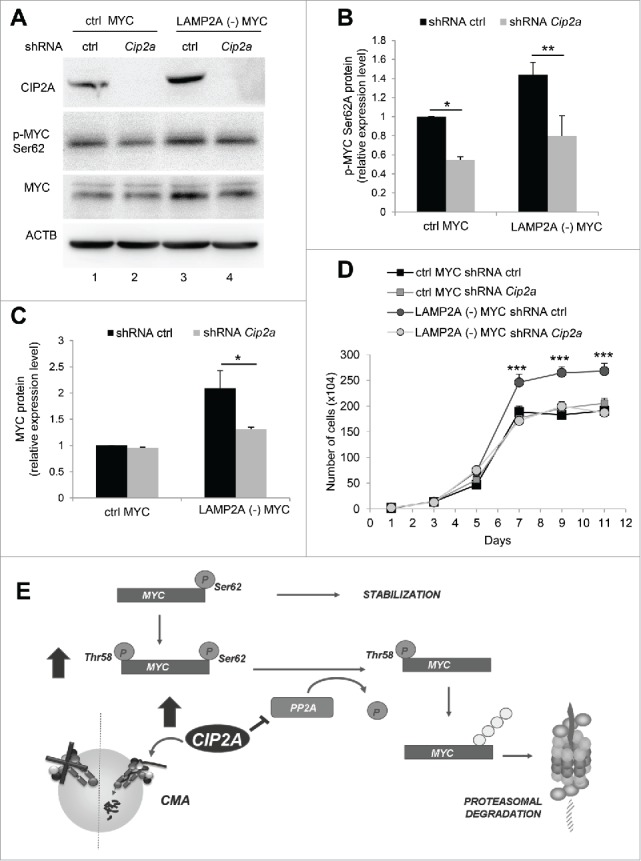
Increased CIP2A expression is required for the regulatory effect of CMA on MYC levels. NIH-3T3 cells overexpressing MYC, control (ctrl) or LAMP2A knockdown (LAMP2A [-]) were transduced with lentivirus carrying shRNA against Cip2a. (A) Total extracts from these cells were used for immunoblotting for the indicated proteins. Densitometric quantification of immunoblots as the one in A for phospho-MYC (Ser62) (B) and total MYC (C). Values are relative to those in ctrl cells and normalized to Ponceau S staining (N = 4). (D) Growth curve of these cells (N = 6). (E) Schema of the proposed regulatory effect of CMA on MYC levels: CMA favors activation of the phosphatase PPP2 by directly degrading its endogenous inhibitor CIP2A in lysosomes. Higher PPP2 activity results in dephosphorylation of MYC on Ser62 promoting its subsequent degradation by the proteasome. CMA inhibition leads to CIP2A accumulation and, consequently, decreased PPP2 activity and MYC stabilization by preventing its proteasomal degradation. In the graphs, values are presented as mean ± SEM. Two-way ANOVA and the Bonferroni post-hoc test were used and differences were considered significant for *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
