Abstract
The genetic and physiological mechanisms underlying the relationship between water-soluble carbohydrates (WSC) and water stress tolerance are scarcely known. This study aimed to evaluate the main WSC in stems, and the expression of genes involved in fructan metabolism in wheat genotypes growing in a glasshouse with water stress (WS; 50% field capacity from heading) and full irrigation (FI; 100% field capacity). Eight wheat genotypes (five tolerant and three susceptible to water stress) were evaluated initially (experiment 1) and the two most contrasting genotypes in terms of WSC accumulation were evaluated in a subsequent experiment (experiment 2). Maximum accumulation of WSC occurred 10–20 days after anthesis. Under WS, the stress-tolerant genotype exhibited higher concentrations of WSC, glucose, fructose and fructan in the stems, compared to FI. In addition, the stress-tolerant genotype exhibited higher up-regulation of the fructan 1-fructosyltransferase B (1-FFTB) and fructan 1-exohydrolase w2 (1-FEHw2) genes, whereas the susceptible cultivar presented an up-regulation of the fructan 6-fructosyltransferase (6-SFT) and fructan 1-exohydrolase w3 (1-FEHw3) genes. Our results indicated clear differences in the pattern of WSC accumulation and the expression of genes regulating fructan metabolism between the tolerant and susceptible genotypes under WS.
Introduction
Water deficit is an important abiotic stress factor that limits the growth and productivity of major crop species, including wheat [1]. It affects a large number of physiological processes such as leaf gas exchange capacity [2, 3], timing of phenological phases, partitioning and stem reserve utilization, osmotic adjustment, and accumulation of stress-related proteins and antioxidant defense, among others [2, 4]. Also, water deficit influences morphological and agronomic traits such as leaf area, plant height, total biomass, and seed weight [4, 5].
In wheat, water deficit in Mediterranean climates usually occurs from heading and continues during grain formation (i.e. terminal drought), which reduces both the number of kernels per spike, grain weight and yield [6, 7]. Considering this impact on crop productivity, it is essential to identify robust physiological, biochemical and molecular traits that allow selection of water stress-tolerant genotypes for use in breeding programs [1, 8]. Indeed, several studies have reported genotypic variation in the physiological and agronomic traits associated with water stress tolerance in wheat and other cereals [2, 9, 10, 11]. Plants use different mechanisms to tolerate water stress and avoid damage. One of those present in cereals is the accumulation of water-soluble carbohydrates (WSCs) in the stem and leaf sheath up to anthesis, which are then translocated to the spike and grains during grain filling [12, 13]. The accumulation of WSCs begins when the internodes are elongated from the jointing stage to grain filling, however, the total quantities depend on the genotype and environmental conditions [11, 14]. The highest WSC levels are located between the peduncle and penultimate internode [15, 16, 17].
Among the WSCs are glucose (Glu), fructose (Fru), sucrose (Suc), and fructan, the latter being the dominant form in wheat stems [18, 19]. According to Michiels et al. [20] wheat genotypes that are able to synthesize and store a higher concentration of WSCs in the stems before anthesis are more likely to exhibit improved grain yield under water stress conditions. This is because the photosynthetic carbon assimilation during post-anthesis is inhibited by water stress conditions, therefore grain growth and filling depend more on stem reserves that are mobilized to the grain [21, 22, 23]. Indeed, translocation of WSCs from the stems could be responsible for 10–20% of the grain yield in irrigated crops [14, 24] and 40–60% under severe water stress conditions during the grain-filling period [21]. Nevertheless, the relationship between stem WSC concentration or content and grain yield (GY) in wheat is not clear, since some studies have found positive relationships [25], but others have reported no significant relationships or even negative relationships [11, 26].
Fructans can account for up to 85% of WSCs in the wheat stem internodes at the stage of maximum accumulation [15, 19, 27], while Suc represents only 10% [28, 29]. Fructans are linear or branched polymers that are synthesized from Suc [30], and they vary in length from trisaccharides (1-ketotriose, 6-ketotriose, and neo-ketotriose) to polysaccharides that have hundreds of Fru units [31]. Fructans, whether linear or branched, are formed by Fru molecules and often by terminal Glu [32]. In wheat, fructans are mixed levans (graminan-type) composed by both (2–1)- and (2–6)-linked β-D-fructosyl units [33]. Genotypic differences in stem WSC concentrations are mainly attributed to fructans [12, 18].
Fructan biosynthesis is mediated by four fructosyltransferase (FT) enzymes [32, 34]: 1-SST (sucrose:sucrose 1-fructosyltransferase); 1-FFT (fructan:fructan 1-fructosyltransferase); 6-SFT (sucrose:fructan 6-fructosyltransferase); and 6G-FFT (fructan:fructan 6G-fructosyltransferase). In wheat, 1-SST produces the trisaccharide, 1-ketotriose (1-K), and Glu from two Suc molecules, 6-SFT uses 1-K as a substrate to produce 1- and 6-ketotriose (1-K and 6-K, a branched tetrasaccharide), and 1-FFT and 6-SFT are involved in chain elongation [12, 35]. The enzyme 6-SFT transfers a Fru unit from a Suc unit to a Fructan by β (2,6) linkages [36]. In the graminae, Bromus pictus, the expression of the 6-SFT enzyme is accompanied by accumulation [37], and in Lactuca sativa, the increase in plant fructan content is directly related to an increase in the WSC content [38]. In wheat, the expression of the 1-SST and 6SFT genes in the stem was positively correlated with stem WSCs and fructan concentrations [12].
The mobilization of stored carbohydrates requires fructan hydrolysis, which is catalyzed by fructan exohydrolase (FEH) enzymes [12]. These include 1-fructan exohydrolase (1-FEH) and 6-fructan exohydrolase (6-FEH) [39, 40, 41] and they catalyze the reaction of fructan, which participates in fructan depolymerization, with β (2,1) and β (2,6) linkages, respectively [42]. The β (2,6) linkages are predominant in wheat stems [35, 43].
As far we are aware, there is little information on how the WSC is accumulated and mobilized from the stem to the grains after anthesis, and how this process is affected by drought stress. Also, the gene expression patterns of stem enzymes responsible for fructan accumulation and remobilization in wheat genotypes growing under water stress and well-irrigated conditions are little understood. A recent study from Zhang et al. [26] showed that high expression of the 1-FEH w3 gene contributed to the high levels of fructan and stem WSC remobilization to the grains in bread wheat under drought conditions. Another study by Cimini et al. [34] indicated that the accumulation of fructans in grains of durum wheat growing in field conditions was closely associated with the gene expression and activity of fructan biosynthetic enzymes (mainly 1-SST and 1-FFT).
This study aimed to evaluate the effect of water stress on the concentration of WSCs in stems and the expression of genes involved in fructan metabolism from anthesis to maturity in wheat genotypes with different tolerances to water stress. The genotypes were selected from a large set of 384 cultivars and advanced lines of spring wheat, which were evaluated in the field under water stress and fully irrigated conditions, during two growing seasons [11]. We hypothesize that genotypes with contrasting drought tolerance exhibit different WSC and gene expression dynamics when they are subjected to water deficit.
Materials and methods
Plant material and growth conditions
Eight contrasting genotypes were selected according to the yield tolerance index (YTI) from a study conducted under field conditions where water deficit tolerance was evaluated in 384 cultivars and advanced lines of spring wheat [11]. Two experiments were conducted under greenhouse conditions at the Instituto de Investigaciones Agropecuarias (INIA, Institute of Agricultural Research) Quilamapu, Chillán (36°31' S; 71°54' W), Chile in the 2013–2014 and 2014–2015 growing seasons. Eight wheat genotypes (five tolerant and three susceptible to water stress) were evaluated for WSC accumulation in the first experiment (experiment 1) and two contrasting genotypes in terms of grain yield under water stress conditions in field experiments [11] and stem carbohydrate accumulation were evaluated in the second experiment (experiment 2) (Table 1). Greenhouse conditions were 12 h light at 22°C and 55% to 60% relative humidity. Seeds were sown in 5 l pots (4 seeds per pot) in a substrate mixture of loam soil, vermiculite and sand, in a ratio of 5.5: 2.0: 2.5 (v/v), respectively. The physical and chemical properties of the selected soil were: 48 ppm N, 19.91 ppm P, 388.3 ppm K, and pH 6.15. Additionally, at sowing the plants were fertilized with Basacote Plus 3M (COMPO, Münster, Germany), which is a controlled-release fertilizer, and it was applied at a rate of 3 g l-1 substrate.
Table 1. Genotypes evaluated in experiments 1 and 2, and their level of tolerance to water stress under field conditions, according to the yield tolerance index (YTI).
| Genotype | Origin | YTI1 | Tolerance to stress |
|---|---|---|---|
| Experiment 1 | |||
| FONTAGRO 8 | INIA-Chile | 0.60 | Tolerant |
| Pantera | INIA-Chile | 0.49 | Tolerant |
| Don Alberto | INIA-Uruguay | 0.47 | Tolerant |
| LE2384 | INIA-Uruguay | 0.43 | Tolerant |
| QUP2522 | INIA-Chile | 0.35 | Tolerant |
| Fontagro 69 | INIA-Chile | 0.23 | Susceptible |
| CCCI09 | INIA-Uruguay | 0.20 | Susceptible |
| Fontagro 98 | CIMMYT-Mexico | 0.18 | Susceptible |
| Experiment 2 | |||
| LE 2384 | INIA-Uruguay | 0.43 | Tolerant |
| Fontagro 69 | INIA-Chile | 0.23 | Susceptible |
1: According to del Pozo et al. [11]; higher values indicate more tolerance to water stress.
In experiment 1, seeds were sown on 31 July 2013. Two irrigation treatments were established from heading (Zadoks stage Z5.5) [44]: 50% (water stress; WS) and 100% (full irrigation; FI) of field capacity. Before heading, all the plants were grown to field capacity. Fifteen pots were established for each genotype and treatment.
In experiment 2, seeds were sown on 16 July 2014 and the same water treatments as in experiment 1 were established. Soil water content was evaluated by 10HS sensors (Decagon Devices, USA) connected to an EM-50 data logger (Decagon Devices, USA). The 10HS sensor determines volumetric water content by measuring the dielectric constant of the soil using frequency domain capacitance technology. Eight humidity sensors were available, two for each genotype and treatment, and data are shown in S1 Fig.
Analysis of WSCs, Glu, Fru, Suc, and Fruct
Water-soluble carbohydrates were evaluated in experiments 1 and 2 from anthesis (Z6.5) to physiological maturity (Z9.0). Samples were taken from two primary stems, with four replicates, at different developmental stages from anthesis to maturity, at 10-day intervals in experiment 1 and 7-day intervals in experiment 2. The WS treatment started at heading in each genotype and this was approximately ten days before anthesis (S1 Fig). On each sample date, two stems were cut (between the penultimate and ultimate node) and mixed; half of them were dried at 65°C for 48 h and used for measuring WSCs and the other half were stored at -80°C for quantifying FT and FEH gene expression. The anthrone (Merck, Germany) method was used to determine WSC concentration [45]. The Glu, Fru, Suc, and fructan contents were determined in experiment 2, in the two contrasting genotypes, in terms of WSC content. The powdered samples were extracted in buffer that contained 80% ethanol and 10mM HEPES-KOH pH 7.4 and incubated at 70°C for 2 h with shaking. After centrifugation for 30 min at 10000 rpm at room temperature, the supernatant was stored at -20°C. The pellet was used for further extraction by adding 2000 μl of extraction buffer, incubating at 65°C for 24 h with continuous shaking and centrifuging at 10000 rpm for 15 min. The supernatant was collected and added to the previously stored sample. Samples were clarified using Carrez Reagent 1: 85 mM potassium hexacyanoferrate ferrocyanide, and Carrez Reagent 2: 85mM, zinc sulfate until the metabolites were quantified for 100 samples with the Sucrose, D-Glucose, and D-Fructose K-SUFRG enzymatic assay kit (Megazyme, Ireland) according to the manufacturer’s protocol (K-SUFRG 06/14).
For fructan measurement 100 mg of powder samples were extracted using 40 ml of milli-Q water at 80°C for 20 min. Fructan quantification was performed using the Megazyme Fructan assay kit (Megazyme International, Ireland), according to the manufacturer’s description.
Physiological traits
Chlorophyll content (SPAD index), relative water content (RWC), and stomatal conductance (gs) in the flag leaves were evaluated in experiment 2 from the anthesis stage (Z6.5) to the start of the grain dough stage (Z8) with four replicates. Flag leaf chlorophyll content was measured with a SPAD 52 portable chlorophyll meter (Minolta Spectrum Technologics Inc., Plainfield, IL, USA). To measure RWC, fresh flag leaf samples were weighed, submerged in distilled water for 24 h at 4°C, and finally dried at 65°C for 48 h. Relative water content was calculated as: RWC = [(FW-DW) x 100] / (TW-DW), where FW, DW and TW are the fresh, dry and turgid leaf weights, respectively. Stomatal conductance (gs) was measured in the flag leaves with a leaf porometer (model SC-1, Decagon Devices, Pullman, WA, USA).
Agronomic traits
The following traits were evaluated at physiological maturity: plant grain yield (GY), number of kernels per spike (NKS), 1000-grain weight (TKW), number of spikelets per spike (NSS), number of kernels per spike (KPS), and plant dry matter (DW). Evaluations were performed in two plants per pot, and in four replicates.
Gene expression analyses
The expression of the FT and FEH genes was evaluated by quantitative RT-PCR (qRT-PCR). Extraction of RNA was carried out from wheat stems using the SV Total RNA System kit (Promega, Madison, WI, USA). The quality of RNA was evaluated by denaturing gel electrophoresis and quantification was performed by measuring the absorbance at 260 and 280 nm in a spectrophotometer (EPOCH, Biotek, VT, USA). The cDNA was synthesized using the Superscript reverse transcriptase system (Invitrogen, Carsbad, CA, USA) from 500 ng of total RNA. Specific primers were designed for the genes, 1-SST, 1-FFTA, 1-FFTB, 6-SFT, 1-FEHw1, 1-FEHw2, 1-FEHw3, and 6-FEH using Primer Quest and the Oligoanalyzer platform (https://www.idtdna.com/calc/analyzer). The sequences of the primers utilized for amplifying the target and reference (α-tubulin) genes are indicated in S1 Table. Primers were evaluated according to Czechowski et al. [46]. Real time PCR was performed using the 5x HOT FIREPol, EvaGreen qPCR Mix Plus Kit (Biotium, USA) in an Eco Real-Time PCR thermocycler (Illumina, USA). All qRT-PCRs were normalized with threshold cycle (Ct) values of the reference gene. The expression variation for the selected genes was estimated as described below. Evaluations were performed at d0, d7, d14 and d21. Samples in subsequent stages could not be obtained due to accelerated senescence of sensitive genotypes. The values are the mean of three biological and two technical replicates.
Statistical analysis
A factorial experimental design was used to analyze carbohydrates. It combined three factors: genotype (G: ‘Fontagro 69’ and ‘LE 2384’), water treatment (E: WS and WI), and developmental stage (S: days after anthesis; daa). Each experimental unit consisted of a pot with four plants. Two of them were sampled. Analyses of variance (ANOVA) were performed to determine the effects of genotype, environment (water regime), and growth stage (days after anthesis) and their interaction, utilizing the GLM procedure in the SAS package 9.0 [47].
Gene expression was analyzed with the 2–ΔΔCt method described by Yuan et al. (2006) [48]. For any particular gene and day after anthesis, the ΔCt values were calculated as the difference between the expression of the target and the reference gene (α-tubulin). The ΔΔCt values were estimated as the difference between the ΔCt determined under WS or FI conditions and the ΔCt measured under FI and at d0. Analysis of yield components was performed by ANOVA and subsequently by Duncan’s test. Additionally, an ANOVA by physiological stage and genotype was performed to evaluate the effect of genotype and water stress treatment. A correction for false positives associated with the p-values estimated from gene expression analyses was performed utilizing the False Discover Rate [49], using the package qvalue for R [50]. Pearson correlations were also performed between the different analyzed variables to identify association patterns. In particular, the relative expression estimates were analyzed along with physiological traits, and carbohydrate contents, from 0 to 21 daa.
Finally, a principal component analysis (PCA) was developed to analyze simultaneously the assessed variables. All the statistical analyses were performed with SAS-JMP software [47].
Results
Concentration of carbohydrates in the intermediate stem
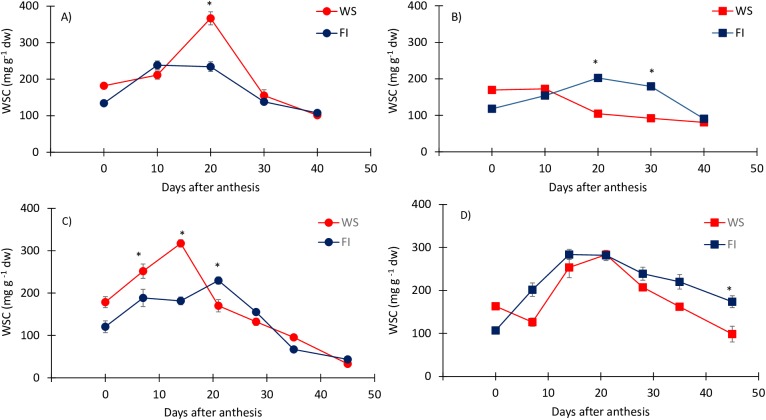
A wide variation was observed in experiment 1 for WSCs in the eight genotypes (Fig 1A). The apparent WSC remobilization (DWSC) under WS conditions, estimated as the difference between the maximum and minimum (at physiological maturity, 40 daa), was linearly related to the yield tolerance index, the latter determined in field conditions (Table 1; Fig 1B). Based on this information, the tolerant genotype with maximum WSC concentration (‘LE 2384’) and the susceptible genotype with the lowest WSCs (‘Fontagro 69’) were selected as contrasting varieties (Fig 1B) for studies of carbohydrate composition and gene expression related to fructan synthesis and degradation.
Fig 1.
Maximum water-soluble carbohydrates (WSCs) in the stem (A) and relationship between the apparent WSC remobilization and the yield tolerance index (YTI) determined in field experiments by del Pozo et al. [11] (B). WSC was assessed from anthesis to maturity on eight tolerant and susceptible genotypes to water deficit grown under water stress (WS) and full irrigation (FI) conditions (experiment 1). The symbols in red are the two contrasting genotypes used in experiment 2.
In genotype ‘LE 2384’, the stem WSCs under WS conditions were at their maximum at 14–20 daa and significantly higher (P<0.05) than under FI conditions, whereas in ‘Fontagro 69’, the WSCs were significantly higher under FI conditions (Fig 2). Thus, the stem WSCs of both cultivars presented a significant (P<0.001) GxExS interaction (Table 2). In experiment 2, the DWSC under WS conditions was also higher for ‘LE 2384’ (273.76 mg g-1; 86.26%) than ‘Fontagro 69’ (188.13 mg g-1; 63.25%). This was also true under FI, where the DWSC was 185.92 mg g-1 and 109.91 mg g-1 for ‘LE 2384’ and ‘Fontagro 69’, respectively.
Fig 2. Variation in the concentration of water-soluble carbohydrates (WSCs) in the stem.
Measurements were performed from anthesis to maturity on the tolerant (‘LE 2384’; A and C) and susceptible (‘Fontagro 69’; B and D) genotypes, grown under water stress (WS) and full irrigation (FI) conditions, in experiment 1 (A and B) and experiment 2 (C and D). Values are the mean ± SE of four replicates, * P <0.05 (according to Duncan’s test).
Table 2. Significance levels from ANOVA performed for the glucose, fructose, sucrose, fructan and water-soluble carbohydrate (WSC) concentration in wheat stems measured in experiment 2.
| Trait | Genotype (G) | Environment (E) | Stage (S) | GxE | GxS | ExS | GxExS |
|---|---|---|---|---|---|---|---|
| Glucose | ** | *** | *** | ** | *** | *** | *** |
| Fructose | ** | ** | *** | * | *** | * | * |
| Sucrose | NS | ** | *** | NS | * | ** | NS |
| Fructan | *** | NS | *** | *** | *** | * | ** |
| WSC | *** | NS | *** | *** | *** | *** | ** |
* P< 0.05
** P< 0.001
*** P< 0.0001. NS: non-significant.
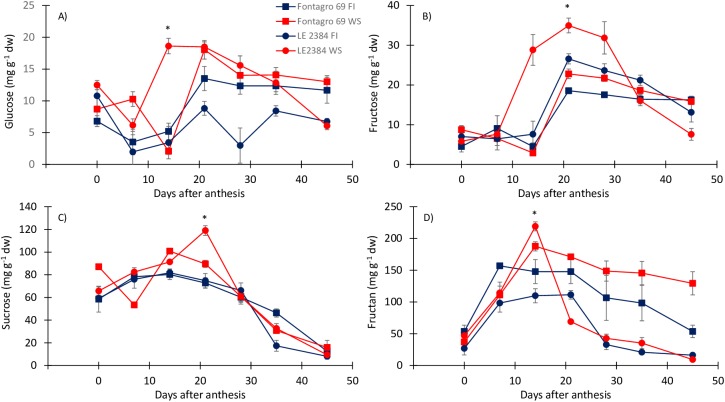
The analysis of Glu, Fru, and fructan concentrations resulted in significant GxExS interactions (P<0.0001, P<0.05, and P<0.01, respectively, Table 2). Water stress increased the concentrations of Glu at 14 daa and Fru between 14 and 28 daa, in genotype ‘LE 2384’ but not in ‘Fontagro 69’ (Fig 3A and 3B). No significant (P>0.05) GxExS interaction was recorded for Suc, however, ‘LE 2384’ had a higher concentration under WS at 21 daa (P<0.05), which explain the significance of the E effect (Fig 3C). Fructans were the predominant WSCs in both cultivars and water conditions, with higher values (P<0.05) at 14 daa under WS for both cultivars, however, they decreased sharply up to 45 daa in ‘LE 2384’ (Fig 4D). As a consequence, ‘LE 2384’ exhibited higher Fructan remobilization under WS conditions (209.65 mg g-1) compared with ‘Fontagro 69’ (58.26 mg g-1).
Fig 3.
Changes in concentration of A) glucose, B) fructose, C) sucrose and D) fructans in the stem, from anthesis to maturity. The tolerant (‘LE 2384’) and susceptible (‘Fontagro 69’) genotypes were grown under water stress (WS) and full irrigation (FI) conditions. Values are the mean ± SE of four replicates, * P <0.05 (according to Duncan’s test).
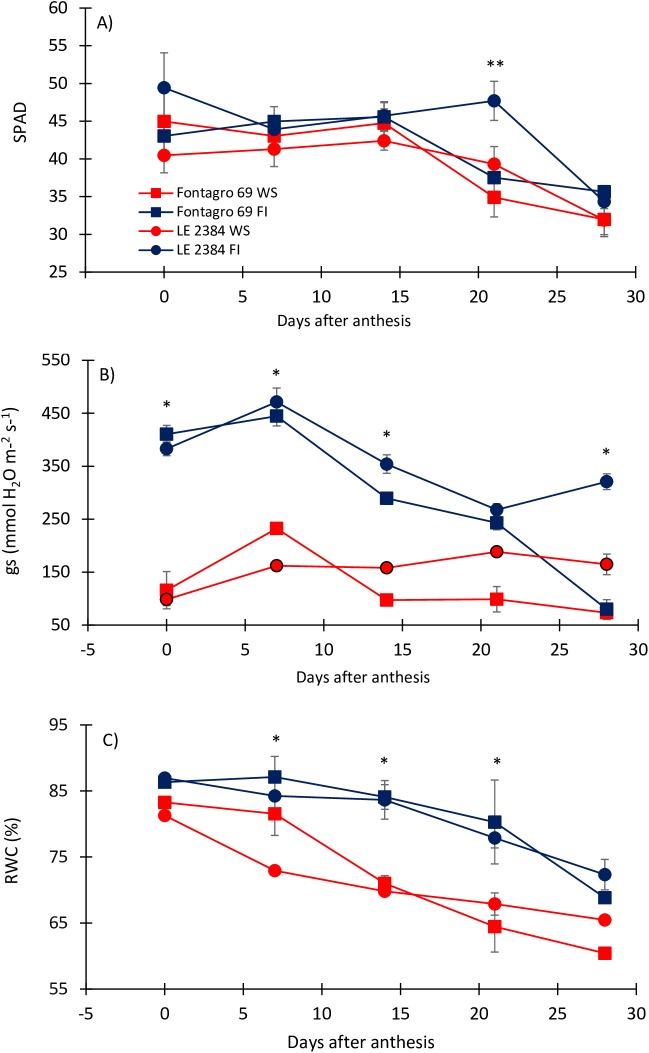
Fig 4.
Changes in A) chlorophyll index (SPAD), B) stomatal conductance (SC), and C) relative water content (RWC) observed from anthesis to maturity. The tolerant (LE 2384) and susceptible (Fontagro 69) genotypes were grown under water stress (WS) and full irrigation (FI) conditions in 2014 (experiment 2). Vertical lines represent ± SE of the means of the four replicates, *significant differences between treatments, (P<0.05) according to Duncan’s test.
Physiological and agronomical traits
The SPAD index, RWC and gs were evaluated to demonstrate the effect of water deficit in both genotypes. The SPAD index was significantly higher in ‘LE 2384’ under FI at 21 daa (P<0.05) (Fig 4A). The RWC and gs were clearly lower under WS conditions in both genotypes (Fig 4B and 4C). The gs was significantly higher during FI compared with WS treatment. Additionally, gs maintained higher values in ‘LE2384’ under WS (until 28 daa), compared with ‘Fontagro 69’ (Fig 4B).
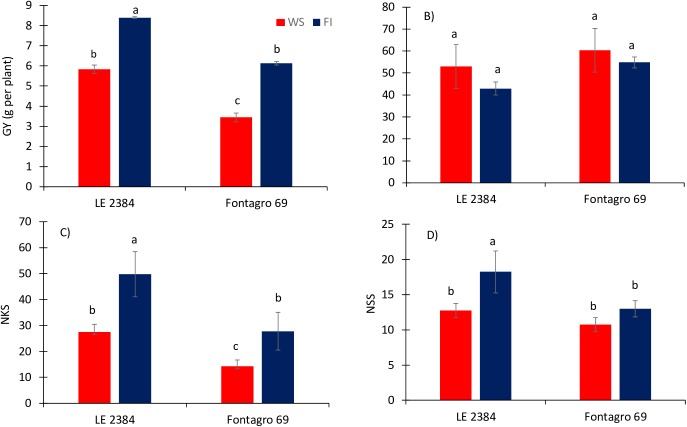
Under WS conditions, GY decreased 30.5% and 43.9% for the genotypes ‘LE 2384’ and ‘Fontagro 69’, respectively (Fig 5A). Water deficit also decreased the number of kernels per spike (NKS) and the number of spikelets per spike (NSS) (Fig 5). Cultivar ‘LE 2384’ had higher GY and NKS under both water regimes (Fig 5A and 5C).
Fig 5. Yield and yield components at physiological maturity for ‘LE 2384’ and ‘Fontagro 69’ genotypes under water stress (WS) and full irrigation conditions (FI).
A) grain yield per plant (GY), B) thousand kernel weight (TKW), C) number of kernels per spike (NKS), and D) number of spikelets per spike (NSS). Values are the mean ± SE of four replicates. The lowercase letters above the bars represent significant differences at P<0.05 according to Duncan’s test.
Analysis of relative gene expression
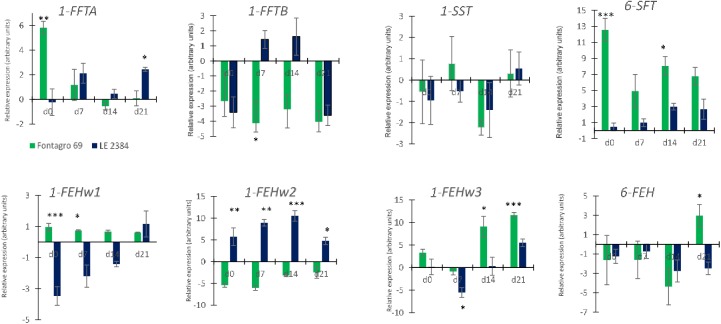
The determination of relative gene expression was conducted from 0 to 21 days after anthesis. After this period, the plants grown under water stress condition got drier and it was not possible to isolate undegraded RNA. Among the FT genes, 1-FFTA was significantly up regulated under WS conditions in ‘Fontagro 69’ at d0 and ‘LE 2384’ at 21 daa (P<0.001 and P<0.05, respectively) (Fig 6A). The 1-FFTB gene was down regulated under WS in ´Fontagro 69´ and up regulated in ‘LE 2384’ at 7 and 14 daa (Fig 6B). The expression of the 1-SST gene was similar for both genotypes with small differences between water treatments (Fig 6C). The 6-SFT gene was up regulated under WS in both genotypes, but the level of expression from anthesis to 21 daa was much higher in ‘Fontagro 69’ (Fig 6D).
Fig 6. Relative expression of genes involved in fructan metabolism in the stem.
The expression of four fructosyltransferase (1-FFTA, 1-FFTB, 1-SST and 6-SFT) and four fructan exohydrolase (1-FEHw1, 1-FEHw2, 1-FEHw3 and 6-FEH) genes was determined in the stems from d0 (anthesis) to 21 days after anthesis (d21), in ‘LE 2384’ and ‘Fontagro 69’ genotypes, grown under water stress (WS) and full irrigation (FI) conditions. Levels of expression were measured by qRT-PCR using primers displayed in Table 2, and the relative expression calculated by 2–ΔΔCt method described in Material and Methods. Vertical lines represent the mean ± SE of three biological replicates, significant differences at P< 0.05*, P<0.001, ** and P<0.0001***.
Three forms of the 1-FEH gene were evaluated: 1-FEHw1 (1-FEH-6A), 1-FEHw2 (1-FEH-6D), and 1FEHw3 (1-FEH-6B). Results showed significant differences between genotypes in the expression of these genes under WS conditions; in ‘LE 2384’ the 1- FEHw1 gene was down regulated and 1-FEHw2 was up regulated, but the opposite occurred in genotype ‘Fontagro 69’ (Fig 6E and 6F). In addition, the 1-FEHw3 gene was induced in ‘Fontagro 69’ at 14 and 21 daa (Fig 6G). Expression of the 6-FEH gene was significantly different between genotypes at 21 daa; the susceptible genotype exhibited up regulation while the tolerant genotype exhibited down regulation (Fig 6H). Correction for false positives indicated q-values under 0.1 for all the observed significant differences between both genotypes (S2 Table).
Pearson correlations indicated that in genotype ‘LE 2384’, the relative expression of the 1-FFTA gene was positively correlated with 6-FEH under WS conditions and with 1-FFTB under FI (Table 3), whereas in ‘Fontagro 69’, it was positively correlated with 1-FFTB and 1-SST under WS, and with 1-FFTB and 6-SFT under FI (Table 4). Also, in genotype ‘LE 2384’, the 1-FEHw1 gene was positively correlated with 1-FEHw3 under WS conditions, but negatively with 1-FEHw2 under FI (Table 3). In ‘Fontagro 69’, the 1-FEHw2 was positively correlated with 1-FEHw3 under WS conditions (Table 4). Under WS conditions, fructan was positively correlated with 6-SFT and 1-FEHw2 genes in genotype ‘LE 2384’, and with 1-FFTA, 1-FEHw2 and 1-FEHw3 in ‘Fontagro 69’. Under FI conditions, fructan was positively correlated with 1-FEHw1 and 1–FEHw3 in ‘LE 2384’, and with 6-SFT and 6-FEH in ‘Fontagro 69’ (Table 4). Fructan presented a high and positive correlation with WSCs in both genotypes and water treatments.
Table 3. Pearson correlation matrix between the relative expression of genes encoding fructosyltransferase and exohydrolases enzymes, and water-soluble carbohydrates.
Data are from evaluations performed at 0, 7, 14 and 21 days after anthesis, for the genotype “LE 2384” grown under water stress (unshaded matrix) and full irrigation conditions (shaded matrix).
| 1-FFTA | 1-FFTB | 1-SST | 6-SFT | 1-FEHw1 | 1-FEHw2 | 1-FEHw3 | 6-FEH | Glu | Fru | Suc | Fruct | WSC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-FFTA | 0,46 | -0,15 | -0,17 | -0,32 | 0,37 | -0,18 | 0,35 | 0,46 | 0,17 | -0,06 | -0,53 | -0,36 | |
| 1-FFTB | -0,11 | -0,36 | -0,37 | 0,43 | 0,02 | 0,02 | 0,25 | 0,34 | 0,25 | 0,19 | -0,31 | -0,09 | |
| 1-SST | 0,25 | 0,33 | 0,45 | 0,22 | -0,12 | -0,49 | -0,33 | -0,66 | -0,68 | 0,11 | -0,05 | -0,29 | |
| 6-SFT | -0,31 | 0,42 | 0,30 | 0,37 | -0,37 | -0,55 | -0,19 | -0,48 | -0,78 | 0,29 | -0,34 | -0,50 | |
| 1-FEHw1 | -0,05 | 0,11 | -0,50 | 0,00 | -0,65 | 0,12 | 0,01 | -0,36 | 0,12 | -0,37 | 0,87 | 0,62 | |
| 1-FEHw2 | -0,25 | 0,14 | 0,11 | 0,29 | -0,14 | -0,26 | -0,17 | 0,25 | -0,07 | 0,18 | -0,73 | -0,55 | |
| 1-FEHw3 | 0,25 | -0,39 | -0,44 | -0,23 | 0,65 | -0,37 | 0,20 | 0,31 | 0,66 | -0,39 | 0,70 | 0,72 | |
| 6-FEH | 0,59 | 0,00 | 0,35 | -0,30 | -0,15 | -0,26 | 0,25 | 0,43 | 0,65 | -0,28 | 0,39 | 0,49 | |
| Glu | -0,36 | -0,14 | 0,20 | 0,10 | -0,47 | -0,18 | -0,24 | 0,11 | 0,79 | -0,11 | -0,06 | 0,28 | |
| Fru | -0,03 | -0,02 | 0,05 | 0,25 | 0,11 | -0,39 | 0,38 | 0,45 | 0,29 | -0,14 | 0,36 | 0,67 | |
| Suc | 0,14 | 0,38 | 0,35 | 0,22 | -0,34 | 0,48 | -0,59 | -0,07 | -0,10 | -0,51 | -0,26 | 0,02 | |
| Fruct | -0,34 | -0,26 | 0,26 | 0,80 | 0,13 | 0,58 | -0,33 | -0,40 | -0,03 | 0,19 | 0,10 | 0,87 | |
| WSC | -0,31 | 0,32 | 0,37 | 0,85 | -0,01 | 0,56 | -0,43 | -0,28 | 0,12 | 0,25 | 0,27 | 0,95 |
Values in bold face are significant at P<0.05.
1-FFTA: fructan 1-fructosyltransferase A, 1-FFTB: fructan 1-fructosyltransferase B, 1-SST: sucrose 1-fructosyltransferase
6-SFT: sucrose 6-fructosyltransferase, 1-FEHw1: fructan 1-exohydrolase w1, 1-FEHw2: fructan 2-exohydrolase w2
1-FEHw3: fructan 3-exohydrolase w3, 6-FEH: fructan 6-exohydrolase, Glu: glucose, Fru: fructose, Suc: sucrose, Fruct: fructan
WSCs: water-soluble carbohydrates.
Table 4. Pearson correlation matrix between the relative expression of genes encoding fructosyltransferase and exohydrolases enzymes, and water-soluble carbohydrates.
Data are from evaluations performed at 0, 7, 14 and 21 days after anthesis, for the genotype “Fontagro 69” grown under water stress (unshaded matrix) and full irrigation conditions (shaded matrix).
| 1-FFTA | 1-FFTB | 1-SST | 6-SFT | 1-FEHw1 | 1-FEHw2 | 1-FEHw3 | 6-FEH | Glu | Fru | Suc | Fruct | WSC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-FFTA | 0,48 | 0,35 | 0,74 | -0,09 | -0,13 | -0,33 | 0,32 | 0,32 | 0,38 | 0,36 | 0,89 | 0,86 | |
| 1-FFTB | 0,63 | 0,47 | 0,03 | 0,37 | 0,18 | -0,17 | 0,02 | 0,30 | 0,29 | 0,15 | 0,26 | 0,29 | |
| 1-SST | 0,52 | 0,55 | 0,09 | 0,04 | 0,13 | -0,38 | -0,27 | -0,11 | -0,44 | 0,11 | 0,07 | 0,02 | |
| 6-SFT | 0,37 | 0,48 | 0,11 | -0,33 | -0,08 | 0,01 | 0,26 | 0,11 | 0,22 | 0,42 | 0,72 | 0,70 | |
| 1-FEHw1 | -0,03 | -0,10 | 0,13 | 0,07 | 0,03 | -0,37 | -0,06 | 0,25 | 0,08 | -0,46 | -0,46 | -0,44 | |
| 1-FEHw2 | 0,31 | 0,09 | -0,28 | 0,22 | 0,15 | 0,06 | 0,05 | 0,03 | 0,15 | -0,33 | -0,23 | -0,23 | |
| 1-FEHw3 | 0,23 | 0,01 | -0,06 | -0,22 | 0,14 | 0,74 | -0,43 | -0,21 | -0,12 | 0,07 | -0,35 | -0,30 | |
| 6-FEH | 0,23 | 0,23 | 0,24 | -0,22 | 0,12 | 0,32 | 0,69 | 0,42 | 0,73 | 0,07 | 0,62 | 0,63 | |
| Glu | -0,37 | -0,22 | 0,12 | -0,14 | 0,68 | -0,16 | 0,21 | 0,53 | 0,65 | 0,02 | 0,41 | 0,48 | |
| Fru | 0,13 | -0,05 | 0,09 | 0,00 | 0,42 | 0,36 | 0,66 | 0,86 | 0,66 | 0,28 | 0,50 | 0,61 | |
| Suc | 0,27 | 0,56 | 0,06 | 0,50 | -0,08 | 0,39 | 0,20 | 0,21 | -0,06 | 0,13 | 0,41 | 0,56 | |
| Fruct | 0,62 | 0,28 | 0,16 | 0,06 | -0,12 | 0,55 | 0,56 | 0,47 | -0,28 | 0,33 | 0,41 | 0,97 | |
| WSC | 0,61 | 0,42 | 0,20 | 0,33 | -0,03 | 0,46 | 0,35 | 0,39 | -0,21 | 0,32 | 0,65 | 0,89 |
Values in bold face are significant at P<0.05.
1-FFTA: fructan 1-fructosyltransferase A, 1-FFTB: fructan 1-fructosyltransferase B, 1-SST: sucrose 1-fructosyltransferase
6-SFT: sucrose 6-fructosyltransferase, 1-FEHw1: fructan 1-exohydrolase w1, 1-FEHw2: fructan 2-exohydrolase w2
1-FEHw3: fructan 3-exohydrolase w3, 6-FEH: fructan 6-exohydrolase, Glu: glucose, Fru: fructose, Suc: sucrose, Fruct: fructan
WSCs: water-soluble carbohydrates.
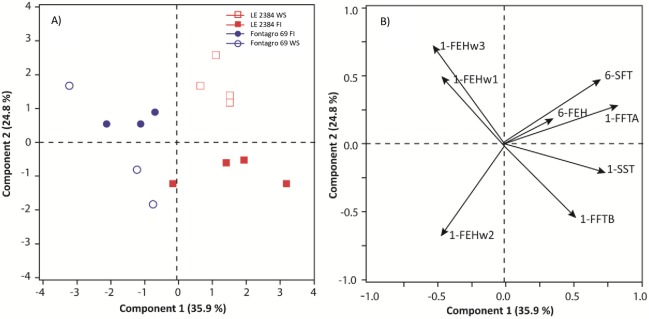
Principal component analysis revealed a clear differentiation between both genotypes along component 1, and between water regimens along component 2 (Fig 7A). Component 1 explained 35.9% of the variation and was positively and significantly associated with the expression of genes 1-FFTA, 1-SST and 6-SFT. Component 2 explained 24.8% and was associated positively with 1-FEHw3 and negatively with the 1-FEHw2 and 1-FFTB genes (Fig 7B).
Fig 7. Biplots of the principal component analysis of relative expression of fructosyltransferase and fructan exohydrolase genes in stems.
Data are from different days after anthesis (0, 7, 14, and 21), in ‘LE2384’ and ‘Fontagro 69’ genotypes, grown under water stress (WS) and full irrigation (FI) conditions. In A) each point is a sampling date (two sampling dates are superimposed in ´Fontagro 69´). In B) 1-FFTA is fructan 1-fructosyltransferase A; 1-FFTB is fructan 1-fructosyltransferase B; 1-SST is sucrose 1-fructosyltransferase; 6-SFT is sucrose 6-fructosyltransferase; 1-FEHw1 is fructan 1-exohydrolase w1; 1-FEHw2 is fructan 2-exohydrolase w2; 1-FEHw3 is fructan 3-exohydrolase w3; and 6-FEH is fructan 6-exohydrolase.
Discussion
Grain yield and its components were higher in the tolerant genotype (‘LE 2384’) under WS and FI conditions. Furthermore, the reduction in GY caused by water stress was less pronounced in the tolerant genotype (Fig 5). This agrees with what we found under water stress conditions in a Mediterranean environment, where LE 2384 yielded 2.3 and 4.9 t ha-1 and ‘Fontagro 69’ yielded 1.0 and 2.5 t ha-1, in 2011 and 2012, respectively [11].
Although the two genotypes differed in flowering date the water stress treatment was initiated at the same growing stage (Z5.5), therefore both genotypes were exposed to water stress from heading to maturity and to similar temperature and light conditions in the greenhouse. Indeed, the RWC and gs values, determined in flag leaves during grain growth, clearly indicate that both genotypes were under similar water stress during grain growth (Fig 4). Also, the tolerant genotype had higher gs and RWC values than the susceptible one during the last developmental stages (Fig 4). A number of authors have reported positive relationships between RWC and yield in wheat [51, 52], indicating that RWC can be used as a selection criterion for drought tolerance in cereals [53].
Grain filling is the final growth stage of cereals and is a crucial stage for economic performance. During this time the WSCs stored in the wheat stem are mobilized and converted into grains [14, 18, 29, 54, 55]. Under water stress conditions, mobilization of WSCs and fructan from the stem to the grain is vital for GY because they can compensate for the negative effect of reduced grain production [14, 15, 26, 27]. Our results indicate that under water stress conditions the tolerant genotype was able to accumulate and remobilize more WSCs and Fructans than the susceptible one. The highest mobilization efficiency recorded for the genotypes under WS conditions, especially ‘LE 2384’, could be mainly due to the reduction in high and low molecular weight fructans in the stem [56, 57].
Fructans are the sugars that predominated in the stems during grain growth. Under WS conditions, the highest concentration of fructans was reached at 14 daa (Fig 3). Another study [58] has also reported maximum values between 8–16 daa. Significant differences in Glu and Fru stem concentrations were observed between the two genotypes; under WS conditions the tolerant genotype had higher concentrations, with their maximum occurring at an earlier stage of grain growth, and they had larger differences with respect to FI (Fig 3). The maximum Suc concentration under WS conditions occurred after (tolerant genotype) or simultaneously (susceptible genotype) with the fructan peak (Fig 3). These results suggest that after anthesis fructans can be converted to Suc, which can effectively compensate for the low photosynthetic supply under water stress conditions, and in this way sustain the grain-filling rate [59].
The results of gene expression showed that the tolerant and susceptible genotypes have differential mechanisms to respond to WS (Tables 3 and 4; Fig 6). In the tolerant genotype ‘LE 2384’, the higher up regulation of fructosyltransferase genes (1-FFTA and 1-FFTB) after anthesis under WS seems to be very important. Also, the up regulation of 6-SFT increased in ‘LE 2384’ as grain filling progressed, and it was positively correlated (r = 0.80) with the fructan concentration in the stem (Table 3; Fig 6). In contrast, ‘Fontagro 69’ exhibited greater levels of up regulation of the 1-FFTA gene at anthesis (d0) and also a high correlation with fructan under WS conditions (Table 4). The down regulation of 1-FFTB in the susceptible cultivar could be related to the catalyzation of fructan synthesis in wheat; Li et al. [57] had previously found that 1-SST, 1-FFTA, and 1-FFTB could be responsible for this process. This could explain the lower fructan content found in the susceptible cultivar.
The fructan exohydrolase enzymes in wheat are responsible for the degradation of fructan into Fru and Suc when the carbohydrate supply is lower than the demand [23, 60]. High expression levels of the 1-FEH isoform are associated with graminan degradation, which is necessary to maintain the carbon flow required for grain filling [61], [62]. Under WS conditions, the relative expression of the 1-FEHw2 gene in genotype ‘LE 2384’ was positively correlated with Suc (Table 3), and in ‘Fontagro 69’, the 1-FEHw2 and 6-FEH genes were positively correlated with Fru (Table 4).
The principal component analysis showed a clear separation between the two cultivars. The tolerant genotype (‘LE 2384’) was associated with the relative expression level of 1-FEHw2, whereas ‘Fontagro 69’ was associated with the expression of the 1-FEHw1 and 1-FEHw3 genes. Therefore, the high upregulation of 1-FEHw2 in the tolerant genotype could be associated with the carbon flow required at grain filling under WS conditions (Fig 6). Also, higher up regulation of the 1-FEHw3 gene was observed in the susceptible genotype (‘Fontagro 69’), but its expression level in ‘LE 2384’ increased at 21 daa, and probably continued increasing under water stress beyond 21 days after anthesis due to the genotype’s late phenology (Fig 6). A study conducted in two wheat cultivars with high yield and stem WSC content revealed that the cultivar with the greatest expression of the 1-FEHw3 gene presented accelerated remobilization of stem WSCs under water stress conditions [61]. The 6-FEH gene was down regulated under WS conditions in both genotypes, except in ‘Fontagro 69’at 21 daa. According to Dreccer et al. [23] and Chen et al. [29], 6-FEH is not inhibited by Suc and this suggests that it might not be involved in mobilizing the reserves, although earlier studies suggested that the most important enzyme in the stem under WS conditions is 6-FEH.
In conclusion, the wheat genotypes ‘LE 2384’ and ‘Fontagro 69’, which have contrasting tolerance to water stress, have different physiological and genetic mechanisms to deal with drought conditions. Our results suggest that the selection of genotypes with higher fructan and WSC remobilization efficiencies would lead to cultivars with higher grain yield under WS conditions. The 1-FFTA, 1-FFTB and 1-SST genes influence fructan synthesis, with a differential regulation under water stress. In relation to the FEH genes, the expression of the three 1-FEH members that were assessed seems to depend on the specific mechanisms used by each genotype to deal with water stress. The 1-FEHw2 gene was possibly associated with sugar translocation from the stem to the grains in the tolerant genotype. Further studies, for example wide genome association analyses or the functional evaluation of candidate genes, will complement our current knowledge and support the development of selection tools for improving productivity under the projected climate change scenarios.
Supporting information
Soil volumetric water content (m3 m-3) in water stress (WS) and full irrigation (FI) in genotype A) ‘LE 2384’ and B) Fontagro 69. Values are the mean of two sensors (replicates). The arrows indicate the anthesis for each genotype.
(DOCX)
Parameter values were determined by the Oligoanalyzer platform (https://www.idtdna.com/calc/analyzer).
(DOCX)
(DOCX)
Acknowledgments
This work was supported by the research grant FONDECYT N° 1150353.
Abbreviations
- 1-FEH
1-fructan exohydrolase
- 1-FFT
fructan:fructan 1-fructosyltransferase
- 1-SST
sucrose:sucrose 1-fructosyltransferase
- 6-FEH
6-fructan exohydrolase
- 6G-FFT
fructan:fructan 6G-fructosyltransferase
- 6-SFT
sucrose:fructan 6-fructosyltransferase
- DW
dry weight
- FEH
fructan exohydrolase
- FI
full irrigation
- Fru
fructose
- Glu
glucose
- gs
stomatal conductance
- GY
grain yield
- KPS
number of kernels per spike
- NKS
number of kernels per spike
- NSS
number of spikelets per spike
- RWC
relative water content
- Suc
sucrose
- TKW
1000-grain weight
- WS
water stress
- WSC
water-soluble carbohydrates
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funded by FONDECYT N° 1150353.
References
- 1.Araus JL, Slafer GA, Royo C, Serret MD (2008) Breeding for yield potential and stress adaptation in cereals. Critical Reviews in Plant Science 27, 377–412. [Google Scholar]
- 2.Monneveux P, Rekika D, Acevedo E, Merah O (2006) Effect of drought on leaf gas exchange, carbon isotope discrimination, transpiration efficiency and productivity in field grown durum wheat genotypes. Plant Science 70, 867–872. [Google Scholar]
- 3.Sikder S, Foulkes J, West H, De Silva J, Gaju O, Greenland A, Howell P (2015) Evaluation of photosynthetic potential of wheat genotypes under drought condition. Photosynthetica 53, 47–54. [Google Scholar]
- 4.Cattivelli L, Rizza F, Badeck FW, Mazzucotelli E, Mastrangelo AM, Francia E, et al. (2008) Drought tolerance improvement in crop plants: an integrated view from breeding to genomics. Field Crops Research 105, 1–2. [Google Scholar]
- 5.Kadam N, Yin X, Bindraban P, Struik P, Jagadish K (2015) Does morphological and anatomical plasticity during the vegetative stage make wheat more tolerant of water-deficit stress than rice? Plant Physiology 167, 1389–401. doi: 10.1104/pp.114.253328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denčić S, Kastori R, Kobiljski B, Duggan B (2000) Evaluation of grain yield and its components in wheat cultivars and landraces under near optimal and drought conditions. Euphytica 113, 43–52. [Google Scholar]
- 7.Estrada-Campuzano G, Slafer GA, Miralles D (2012) Differences in yield, biomass and their components between triticale and wheat grown under contrasting water and nitrogen environments. Field Crops Research 128, 167–179. [Google Scholar]
- 8.Fleury D, Jefferies S, Kuchel H, Langridge P (2010) Genetic and genomic tools to improve drought tolerance in wheat. Journal of Experimental Botany 61, 3211–3222. doi: 10.1093/jxb/erq152 [DOI] [PubMed] [Google Scholar]
- 9.Ji X, Shiran B, Wan J, Lewis DC, Jenkins CLD, Condon AG, et al. (2010) Importance of pre-anthesis anther sink strength for maintenance of grain number during reproductive stage water stress in wheat. Plant, Cell & Environment 33, 926–942. [DOI] [PubMed] [Google Scholar]
- 10.Lobos G, Matus I, Rodriguez A, Romero-Bravo S, Araus JL, del Pozo A (2014) Wheat genotypic variability in grain yield and carbon isotope discrimination assessed by spectral reflectance under Mediterranean conditions. Journal of Integrative Plant Biology 56, 470–479. doi: 10.1111/jipb.12114 [DOI] [PubMed] [Google Scholar]
- 11.del Pozo A, Yáñez A, Matus I, Tapia G, Castillo D, Sanchez-Jardón L, et al. (2016) Field phenotyping of a worldwide germplasm collection of spring wheat under contrasting water conditions in a Mediterranean environment: relationships between physiological and agronomical traits. Frontiers in Plant Science [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xue GP, Mclntyre CL, Colin LD, Jenkins CLD, Glassop D, van Herwaarden AF, et al. (2008) Molecular dissection of variation in carbohydrate metabolism related to water-soluble carbohydrate accumulation in stems of wheat. Plant Physiology 146, 441–45. doi: 10.1104/pp.107.113076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang YP, Zhang YH, Xue QW, Wang ZM (2013) Remobilization of water soluble carbohydrates in non-leaf organs and contribution to grain yield in winter wheat under reduced irrigation. International Journal of Plant Production 7, 97–116. [Google Scholar]
- 14.Dreccer MF, Herwaarden AF, Chapman SC (2009) Grain number and grain weight in wheat lines contrasting for stem water soluble carbohydrate concentration. Field Crops Research 112, 43–54. [Google Scholar]
- 15.Wardlaw IF, Willenbrink J (1994) Carbohydrate storage and mobilization by the culm of wheat between heading and grain maturity: the relation to sucrose synthase and sucrose-phosphate synthase. Functional Plant Physiology 21, 255–272. [Google Scholar]
- 16.Gebbing T (2003) The enclosed and exposed part of the peduncle of wheat (Triticum aestivum)–spatial separation of fructan storage. New Phytologist 159, 245–252. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Chen W, Dell B, Vergauwen R, Zhang X, Mayer JE, Van den End (2015) Wheat genotypic variation in dynamic fluxes of WSC components in different stem segments under drought during grain filling. Frontiers in Plant Science 6: 624 doi: 10.3389/fpls.2015.00624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruuska SA, Rebetzke GJ, van Herwaarden AF, Richards RA, Fettell NA, Tabe L, et al. (2006) Genotypic variation in water-soluble carbohydrate accumulation in wheat. Functional Plant Biology 33, 799–809. [DOI] [PubMed] [Google Scholar]
- 19.Joudi M, Ahmadib Al, Mohadi V, Abbasib A, Vergauwen R, Mohammadi H, et al. (2012) Comparison of fructan dynamics in two wheat cultivars with different capacities of accumulation and remobilization under drought stress. Physiologia Plantarum 144, 1–12. doi: 10.1111/j.1399-3054.2011.01517.x [DOI] [PubMed] [Google Scholar]
- 20.Michiels A, Van Laere A, Van den Ende W, Tucker M (2004) Expression analysis of a chicory fructan 1-exohydrolase gene reveals complex regulation by cold. Journal of Experimental Botany 55, 1325–13333. doi: 10.1093/jxb/erh153 [DOI] [PubMed] [Google Scholar]
- 21.Blum A (1998) Improving wheat grain filling under stress by stem reserve mobilization. Euphytica 100, 77–83. [Google Scholar]
- 22.Plaut Z, Butow BJ, Blumenthal CS, Wrigley CW (2004) Transport of dry matter into developing wheat kernels and its contribution to grain yield under post-anthesis water deficit and elevated temperature. Field Crops Research 86, 185–198. [Google Scholar]
- 23.Dreccer MF, Barnesa L, Mederb R (2014) Quantitative dynamics of stem water soluble carbohydrates in wheat can be monitored in the field using hyperspectral reflectance. Field Crops Research 159, 70–80. [Google Scholar]
- 24.Gebbing T, Schnyder H (1999) Pre-anthesis reserve utilization for protein and carbohydrate synthesis in grains of wheat. Plant Physiology, 121(3), 871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foulkes MJ, Snape JW, Shearman VJ, Reynolds MP, Gaju O, Sylvester-Bradley R, (2007) Genetic progress in yield potential in wheat: recent advances and future prospects. Journal of Agriculture Science 145, 17–29. [Google Scholar]
- 26.Zhang J, Xu Y, Chen W, Dell B, Vergauwen R, Biddulph B, et al. (2015. b). A wheat 1-FEH w3 variant underlies enzyme activity for stem WSC remobilization to grain under drought. New Phytologist 205, 293–305. doi: 10.1111/nph.13030 [DOI] [PubMed] [Google Scholar]
- 27.Goggin DE, Setter TL (2004) Fructosyltransferase activity and fructan accumulation during development in wheat exposed to terminal drought. Function Plant Biology 31, 11–21. [DOI] [PubMed] [Google Scholar]
- 28.Cruz-Aguado JA, Rodes R, Pérez IP, Dorado M (2000) Morphological characteristics and yield components associated with accumulation and loss of dry mass in the internodes of wheat. Field Crops Research 66, 129–139. [Google Scholar]
- 29.Chen W, Xi-Ping D, Sang-Soo E, Egrinya A (2015) The relationship between yield and fructan exo-hydrolases activity in two drought resistant wheat cultivars grown under different fertilizer and tillage treatments. Journal of Plant Nutrition 38, 13–27. [Google Scholar]
- 30.Hisano H, Kanazawa A, Yoshida M, Humphreys MO, Iizuka M, Kitamura K, et al. (2008) Coordinated expression of functionally diverse fructosyltransferase genes isassociated with fructan accumulation in response to low temperature in perennial ryegrass. New Phytologyst 178, 766–780. [DOI] [PubMed] [Google Scholar]
- 31.Suarez-González E, López M, Délano-Frierb D, Gomez-Leyva J (2014) Expression of the 1-SST and 1-FFT genes and consequent fructan accumulation in Agave tequilana and A. inaequidens is differentially induced by diverse (a) biotic-stress related elicitors. Journal of Plant Physiology 171, 359–372. doi: 10.1016/j.jplph.2013.08.002 [DOI] [PubMed] [Google Scholar]
- 32.Lammens W, Le Roy K, Yuan S, Vergauwen R, Rabijns A, Van Laere A, et al. (2012) Crystal structure of 6-SST/6-SFT from Pachysandra terminalis, a plant fructan biosynthesizing enzyme in complex with its acceptor substrate 6-kestose. The Plant Journal 70, 205–219. doi: 10.1111/j.1365-313X.2011.04858.x [DOI] [PubMed] [Google Scholar]
- 33.Vijn I, Smeekens S (1999) Fructan: more than a reserve carbohydrate? Plant Physiology 120, 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cimini S, Locato V, Vergauwen R, Paradiso A, Cecchini C, Vandenpoel L, et al. (2015) Fructan biosynthesis and degradation as part of plant metabolism controlling sugar fluxes during durum wheat kernel maturation. Frontiers in Plant Science 6, 89 doi: 10.3389/fpls.2015.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshida M, Kawakami A, Van den Ende W (2007) Graminan metabolism in cereals: wheat as a model system In: Shiomi N, Benkeblia N, Onodera S (eds) Recent Advances in Fructo oligosaccharides Research. Research Signpost, Trivandrum: pp 201–212. [Google Scholar]
- 36.Tamura K I, Kawakami A, Sanada Y, Tase K, Komatsu T, Yoshida M (2009) Cloning and functional analysis of a fructosyltransferase cDNA for synthesis of highly polymerized levans in timothy (Phleum pratense L.). Journal of Experimental Botany 60, 893–905. doi: 10.1093/jxb/ern337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.del Viso F, Puebla AF, Hopp HE, Heinz RA (2009) Cloning and functional characterization of a fructan 1-exohydrolase (1-FEH) in the cold tolerant Patagonian species Bromus pictus. Planta 231, 13–25. doi: 10.1007/s00425-009-1020-5 [DOI] [PubMed] [Google Scholar]
- 38.Li HJ, Yang AF, Zhang XC, Gao F, Zhang JR (2007) Improving freezing tolerance of transgenic tobacco expressing-sucrose sucrose 1-fructosyltransferase gene from Lactuca sativa. Plant Cell, Tissue and Organ Culture 89, 37–48. [Google Scholar]
- 39.Van Riet L, Nagaraj V, Van den Ende W, Clerens S, Wiemken A, Van Laere A (2006) Purification, cloning and functional characterization of a fructan 6-exohydrolase from wheat (Triticum aestivum L.). Journal of Experimental Botany 57, 213–223. doi: 10.1093/jxb/erj031 [DOI] [PubMed] [Google Scholar]
- 40.Van Riet L, Altenbach D, Vergauwen R, Clerens S, Kawakami A, Yoshida M, et al. (2008) Purification, cloning and functional differences of a third fructan 1-exohydrolase (1-FEHw3) from wheat (Triticum aestivum). Physiologia Plantarum 133, 242–253. doi: 10.1111/j.1399-3054.2008.01070.x [DOI] [PubMed] [Google Scholar]
- 41.De Coninck B, Le Roy K, Francis I, Clerens S, Vergauwen R, Halliday AM, et al. (2005) Arabidopsis AtcwINV3 and 6 are not invertases but are fructan exohydrolases (FEHs) with different substrate specificities. Plant, Cell & Environment 28, 432–443. [Google Scholar]
- 42.Van den Ende W, De Coninck B, Van Laere A (2004) Plant fructan exohydrolase: a role in signaling and defense? Trends in Plant Science 9, 523–528. doi: 10.1016/j.tplants.2004.09.008 [DOI] [PubMed] [Google Scholar]
- 43.Van den Ende W, Clerens S, Vergauwen R, Boogaerts D, Le Roy K, Arckens L, et al. (2006) Cloning and functional analysis of a high DP fructan: fructan 1-fructosyltransferase from Echinops ritro (Asteraceae): comparison of the native and recombinant enzymes. Journal of Experimental Botany 57, 775–789. doi: 10.1093/jxb/erj065 [DOI] [PubMed] [Google Scholar]
- 44.Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for growth stages of cereals. Weed Research 14, 415–421. [Google Scholar]
- 45.Yemm EW, Willis AJ (1954) The estimation of carbohydrates in plant extracts by anthrone. Biochemical Journal 57, 508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR (2005) Genome wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiology 139, 5–17. doi: 10.1104/pp.105.063743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.SAS 9.4. SAS Institute Inc., Cary NC (2013) SAS Institute. JMP Genomics Version 7.1. SAS Institute Inc. Cary NC.
- 48.Yuan YS, Reed A, Chen F, Stewart CN (2006) Statistical analysis of real-time PCR data. BMC Bioinformatics 7, 85 doi: 10.1186/1471-2105-7-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Storey J, Tibshirani R (2003) Statistical significance for genomewide studies. PNAS 100 (16), 9440–9445. doi: 10.1073/pnas.1530509100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bass A, Dabney A, Robinson D (2015) qvalue: Q-value estimation for false discovery rate control. R package version 2.6.0.
- 51.Schonfeld MA, Johnson RC, Carver BF, Mornhinweg DW (1988) Water relations in winter wheat as drought resistance indicator. Crop Science 28, 526–531. [Google Scholar]
- 52.Teulat B, Zoumarou-Wallis N, Rotter B, Salem MB, Bahri H, This D (2003) QTL for relative water content in field-grown barley and their stability across Mediterranean environments. Theoretical and Applied Genetics 108, 181–188. doi: 10.1007/s00122-003-1417-7 [DOI] [PubMed] [Google Scholar]
- 53.Habash DZ, Baudo M, Hindle M, Powers SJ, Defoin-Platel M, Mitchell R, et al. (2014) Systems responses to progressive water stress in durum wheat. PLOSone 9, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ehdaie B, Alloush G, Madore M, Waines J (2006) Genotypic variation for stem reserves and mobilization in wheat: II. Postanthesis changes in internode water-soluble carbohydrates. Crop Science 46: 2093–2103. [Google Scholar]
- 55.Ehdaie B, Alloush GA, Waines JG (2008) Genotypic variation in linear rate of grain growth and contribution of stem reserves to grain yield in wheat. Field Crops Research 106, 34–43. [Google Scholar]
- 56.Wardlaw F, Willenbrink J (2000) Mobilization of fructan reserves and changes in enzyme activities in wheat stems correlate with water stress during kernel filling. New Phytologyst 148, 413–422. [DOI] [PubMed] [Google Scholar]
- 57.Li H, Cai J, Juang D, Liu F, Dai T, Cao W (2013) Flooding stress carbohydrates accumulation and remobilization in wheat plants as influenced by combined waterlogging and shading stress during grain filling. Crop Science 199, 38–48. [Google Scholar]
- 58.Zhang X, Jiang D, Zheng C, Dai T, Cao W (2010) Postanthesis salt and combination of salt and waterlogging affect distributions of sugars, amino acids, Na+ and K+ in wheat. Journal Agronomy and Crop Science 197, 31–39. [Google Scholar]
- 59.Yang J, Zhang J, Wang Z, Zhu Q, Liu L (2004) Activities of fructan- and sucrose-metabolizing enzymes in wheat stems subjected to water stress during grain filling. Planta 220, 331–343. doi: 10.1007/s00425-004-1338-y [DOI] [PubMed] [Google Scholar]
- 60.Verspreet J, Cimini S, Vergauwen R, Dornez E, Locato V, Le Roy K, et al. (2013) Fructan metabolism in developing wheat (Triticum aestivum L.) kernels. Plant and Cell Physiology 54, 2047–2057. doi: 10.1093/pcp/pct144 [DOI] [PubMed] [Google Scholar]
- 61.Zhang JJ, Dell B, Conocono E, Waters I, Setter T, Appels R (2009) Water deficits in wheat: Fructan exohydrolase (1-FEH) mRNA expression and relationship to soluble carbohydrate concentrations in two varieties. New Phytologist 181, 843–850 doi: 10.1111/j.1469-8137.2008.02713.x [DOI] [PubMed] [Google Scholar]
- 62.Kawakami A, Yoshida M (2012) Graminan breakdown by fructan exohydrolase induced in winter wheat inoculated with snow mold. Journal of Plant Physiology 169, 294–302. doi: 10.1016/j.jplph.2011.09.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Soil volumetric water content (m3 m-3) in water stress (WS) and full irrigation (FI) in genotype A) ‘LE 2384’ and B) Fontagro 69. Values are the mean of two sensors (replicates). The arrows indicate the anthesis for each genotype.
(DOCX)
Parameter values were determined by the Oligoanalyzer platform (https://www.idtdna.com/calc/analyzer).
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.