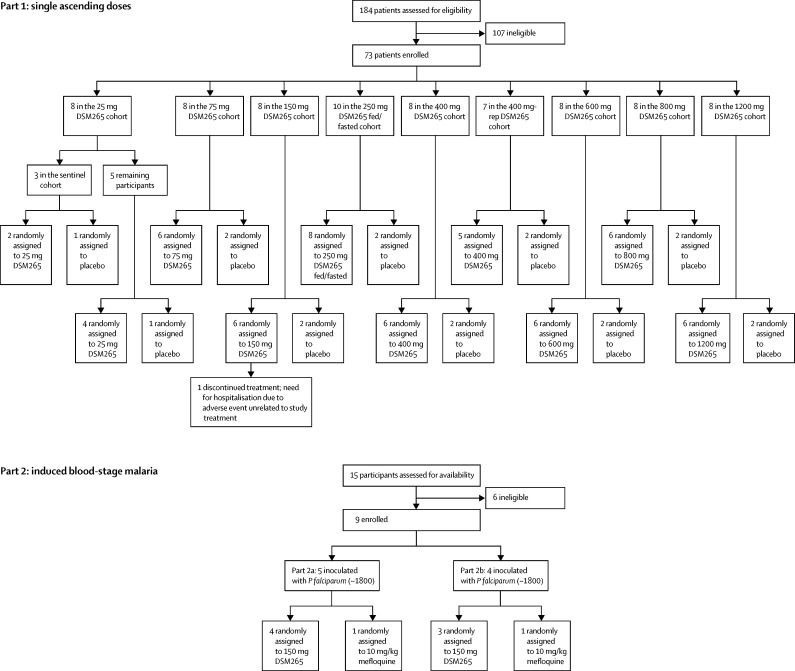
Figure 1.
Trial profile
In part 1, single ascending doses of DSM265 (25–1200 mg) were tested in eight cohorts in fasted conditions. Participants in the 250 mg cohort were to return at least 21 days after the first DSM265 dose to receive DSM265 in fed conditions. The 400 mg dose cohort was repeated (400 mg-rep) due to a biopharmaceutical issue in the preparation of the study medication. Part 2 (induced blood-stage malaria) started after documentation of safety and pharmacokinetics data of the 150 mg dose in part 1.