Abstract
Background:
Saraphi (Mammea siamensis) is a Thai traditional herb. In this study, the cytotoxic effects of crude ethanolic and fractional extracts including hexane, ethyl acetate, and methanol fractions from M. siamensis flowers were investigated in order to determine their effect on WT1 expression in Molt4 and K562 cells and Bcr/Abl expression in K562 cells.
Materials and Methods:
The flowers of M. siamensis were extracted using ethanol. The ethanol flower extract was further fractionated with hexane, ethyl acetate, and methanol. Cytotoxic effects were measured by the MTT assay. Bcr/Abl and WT1 protein levels after treatments were determined by Western blotting. The total cell number was determined via the typan blue exclusion method.
Results:
The hexane fraction showed the strongest cytotoxic activity on Molt4 and K562 cells, with IC50 values of 2.6 and 77.6 μg/ml, respectively. The hexane extract decreased Bcr/Abl protein expression in K562 cells by 74.6% and WT1 protein expressions in Molt4 and K562 cells by 68.4 and 72.1%, respectively. Total cell numbers were decreased by 66.2 and 48.7% in Molt4 and K562 cells, respectively. Mammea E/BB (main active compound) significantly decreased both Bcr/Abl and WTlprotein expressions by 75 and 49.5%, respectively when compared to vehicle control.
Conclusion:
The hexane fraction from M. siamensis flowers inhibited cell proliferation via the suppression of WT1 expression in Molt4 and K562 cells and Bcr/Abl expression in K562 cells. The active compound may be mammea E/BB. Extracts from M. siamensis flowers show promise as naturally occurring anti-cancer drugs.
Keywords: Mammea siamensis, flower, WT1, Bcr/Abl, leukemia, K562, Molt4
Introduction
Saraphi (Mammea siamensis (Miq.) T. Anders.) is a Thai traditional herb and belongs to the Guttiferae family. Its flowers have traditionally been used for heart problems, fever, and enhancement of appetite in Thailand (Poobrasert et. al., 1998). Recent studies of the compounds contain in flower extracts of M. siamensis suggest that the bioactive compounds have significant antiproliferative activities against human leukemia and stomach cancer cell lines (Tung et. al., 2013). The coumarins from the flowers of M. siamensis including kayeassamin A, surangin C, and therapin B demonstrated an antiproliferative action and induced cell apoptosis in HL60 leukemic cells (Tung et. al., 2013). Furthermore, mammea E/BB (an active compound of M. siamensis) exhibited a strong inhibitory effect on WT1 protein expression in leukemic cells (Rungrojsakul et. al., 2016). WT1 is a biological marker for leukemia and is involved in cell proliferation, cell differentiation, and leukemogenesis. The overexpression of WT1 protein has been found in leukemia cells, and the WT1 gene was initially defined as a tumor suppressor gene in pediatric kidney malignancy (Yang et. al., 2007). The normal expression of WT1 involves roles in cell growth and development in hematopoiesis, with expression including the bone marrow and lymph nodes (Menke et. al., 1998). A low level of WT1 protein expression is found in normal blood cells. In contrast, increased levels of WT1 expression are found in leukemic cells, on average 1,000 to 100,000 times higher than in normal blood cells (Inoue et. al., 1994). An inverse correlation between WT1 expression levels and prognosis (Inoue et. al., 1994), increases expression of WT1 at relapse in acute leukemia (Tamaki et. al., 1996) and growth inhibition by WT1 antisense oligomers in leukemic cells (Yamagami et. al., 1996). These results suggest that WT1 plays an important role in leukemogenesis as an oncogene. Bcr/Abl fusion protein is commonly found in chronic myelocytic leukemia (CML) as a biomarker. The Bcr/Abl gene is generated from a reciprocal t(9;22) translocation, known as the Philadelphia chromosome, in which the tyrosine kinase of c-ABL is activated and promotes the growth advantage of leukemic cells (Perez-Caro et. al., 2007). The Philadelphia chromosome (Bcr/Abl fusion protein) is an important biological marker in diagnosing and monitoring cytogenetic response to treatment in CML patients.
The effect of M. siamensis flower extracts on WT1 and Bcr/Abl protein expressions in leukemic cells is as yet unknown. The present study is thus designed to investigate cytotoxic and inhibitory effects of crude ethanolic extract and fractional extracts including hexane, ethyl acetate, and methanol extracts from dried M. siamensis flowers on WT1 expression in Molt4 and K562 cells and Bcr/Abl expression in K562 cells.
Materials and Methods
Plant material
Plant material was collected from February to April, 2014 in Chiang Mai province, Thailand. It was identified by an expert botanist, Mr. James Franklin Maxwell. A voucher specimen is J.F. Maxwell, No. 92-70 and deposits at the CMU herbarium, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand. The flowers were dried in a hot-air oven (45 °C).
M. siamensis flower extraction
The air-dried flowers of M. siamensis were first extracted with ethanol. After removal of the solvent, the obtained crude extract residue was fractionated by quick column chromatography, using a column packed with silica gel, and was partitioned with hexane, ethyl acetate, and methanol to obtain the Hex, EtOAc, and MeOH fractions, respectively. The concentrated solution was completely dried in an evaporator. The four fractions were kept in the refrigerator at -20 °C until use and suspended in DMSO to prepare stock solutions (25,000 μg/ml).
High performance liquid chromatographic (HPLC) analysis
HPLC analyses of crude and fractional extracts of M. siamensis flowers were carried out on a 250 x 4.6 mm, 5 pm (GL Sciences, Inc. Torrance, CA, USA), Inertial ODS-3 column at 25 °C with a flow rate of 0.5 ml/min. The sample run used water with acetic acid (1%) (phase A) and acetonitrile (20/80 v/v) (phase B) and the injection volume was 10 pi The HPLC chromatograms were detected under UV light at 280 nm. Quantitation of the compounds in the extract and in each fraction was performed using mammea E/BB (Rungrojsakul et. al., 2016) as the standard.
Cells and cell cultures
Molt4 (human lymphocytic leukemia cell line) and K562 (human chronic myelocytic leukemia cell line) were used as leukemic cell models in this study, and were cultured in RPMI 1640 medium supplemented with 10% of heat-treated fetal bovine serum, 2 mM L-glutamine, 100 units/ml penicillin and 100 μg/ml streptomycin at 37 °C under a humidified incubator with 5% CO2.
MTT assay
MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was used to evaluate the antiproliferative activities of the crude ethanol and other fractions from the M. siamensis flowers against Molt4 and K562 cells. Each cell line was seeded at a density of 1.0 x 104 cells/well in 96-well plate, and incubated overnight at 37 °C with 5% of CO2. Then, cells were treated with the four extracts (3-100 μg/ml) for 48 h and the complete medium with DMSO was used as a vehicle control (VC). Afterward 15 pl of MTT dye (Sigma-Aldrich, St Louis, MO, USA) solution (5 μg/ml) were added and incubated for another 4 h. The resulting formazan crystals were dissolved with 200 pl of DMSO, and the absorbance was measured at 578 nm with an AccuReader™ microplate reader (Metertech-Inc, Taipei, Taiwan). The reference blank was set at 630 nm. The percentage cell viability was calculated as:
% Cell viability = (Absorbance of test x 100) / (Absorbance of control)
The average cell viability obtained from triplicate experiments was plotted on a graph. The inhibitory concentration at 50% growth (IC50) value was defined as the lowest concentration that decreased cell growth by 50%, whereas the IC20 value was determined as a non-toxic dose and used for protein expression analysis.
Protein extraction and Western blotting
After treatment, leukemic cells were harvested and counted for viable cell numbers using 0.4% typan blue. Thereafter, the cell pellet was lysed by RIPA buffer (25 Mm Tris-HCl, pH 7.6, 150 Mm NaCl, 1% NP-40, 1% sodium deoxycholate (C24H39NaO4), and 0.1% SDS). The protein concentration was evaluated by the Folin-Lowry method. The WT1 protein was separated by 12% SDS polyacrylamide gel electrophoresis and fusion Bcr/Abl protein was separated by 7.5% SDS polyacrylamide gel electrophoresis. The WT1 and Bcr/Abl proteins were detected using primary rabbit polyclonal anti-WT1 (Santa Cruz Biotechnology, CA, USA) or primary rabbit polyclonal anti-Bcr/Abl (USBiological Life Sciences, MA, USA) antibody at 1:1,000 dilution, respectively. GAPDH (Santa Cruz Biotechnology, CA, USA) was used as a loading control with a dilution of 1:1,000. The HRP-conjugated goat anti-rabbit IgG 1:20,000 dilution was used as a secondary antibody. The protein of interest was detected using Luminata™ Forte Western HRP substrate (Millipore Corporation, Billerica, MA, USA) and the protein band levels were quantified by a scanning densitometer and Quantity One software, version 4.6.3 (Bio-Rad laboratories, Hercules, CA, USA).
Statistical analysis
The average of triplicate experiments and standard error of the mean (SEM) were used for quantification. The levels of target protein expression were compared to the vehicle control. Values are shown as mean ± SEM. Differences between the means of each experiment were analyzed by One-way analysis of variance (One-way ANOVA). Differences between mean values were considered statistically significant at p < 0.05.
Results
Cytotoxicity of M. siamensis flower extracts
The cytotoxic effects of fractional extracts on Molt4 and K562 cell lines are shown in Fig. 1. The Hex fraction showed the strongest cytotoxic activity at 48 h in both K562 and Molt4 cells with IC50 values of 2.6 ± 0.1 and 77.6 ± 2.5 μg/ml, respectively (Table 1, Fig. 1a, Fig. 1b). Molt4 cells had a strong response to the Hex fraction treatment than the K562 cells. The EtOAc fraction was less cytotoxic than the Hex fraction while the MeOH fraction had no cytotoxic effects in Molt4 or K562 cells (IC50 > 100 μg/ml).
Figure 1.
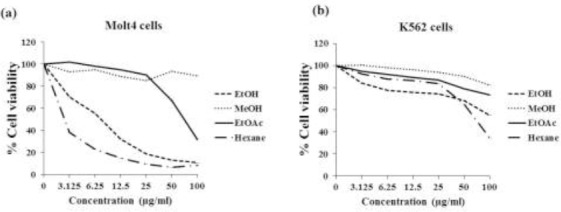
Cytotoxic effect of M. siamensis flower crude EtOH extract and fractional extracts (Hex, EtOAc, and MeOH) against (a) Molt4 and (b) K562 cell lines. The average cell viability was obtained from three independent experiments.
Table 1.
IC50 and IC20 values μg/ml) of M. siamensis flower extracts by MTT assay
| Cells | EtOH | Hex | EtOAc | MeOH | ||||
|---|---|---|---|---|---|---|---|---|
| IC50 | IC20 | IC50 | IC20 | IC50 | IC20 | IC50 | IC20 | |
| Molt4 | 8.4 ± 1.0 | 2.3 ± 0.6 | 2.6 ± 0.1 | 1.0 ± 0.4 | 77.8 ± 1.2 | 36.1± 0.9 | > 100 | > 100 |
| K562 | > 100 | 5.2 ± 0.7 | 77.6 ± 2.5 | 30.6 ± 1.4 | > 100 | 47.2± 0.8 | > 100 | > 100 |
High performance liquid chromatography (HPLC) analysis
The crude EtOH extract and fractional extracts (Hex and EtOAc fractions) were analyzed by HPLC to identify the fingerprint of the flower of M. siamensis and the possible compounds contained in the flower extract. The HPLC fingerprint of mammea E/BB (Rungrojsakul et. al., 2016) was used as a standard marker (Fig. 2a). Crude EtOH extract and the Hex fraction showed two major peaks at the retention times of 36 and 37 min (Fig. 2b, Fig. 2c). The retention time at 36 min indicated mammea E/BB from comparison to the standard mammea E/BB (Fig. 2a), while the retention time of 37 min was reported as an unknown compound peak (Fig. 2b). The EtOAc fraction showed 4 major peaks at the retention times of 5, 5.7, 6.2, and 6.5 min (Fig. 2d) as compared to the crude EtOH extract (Fig. 2b). Among the three extracts, the Hex fraction contained the highest peak of mammea E/BB with the value of 24.38% (Fig. 2c).
Figure 2.
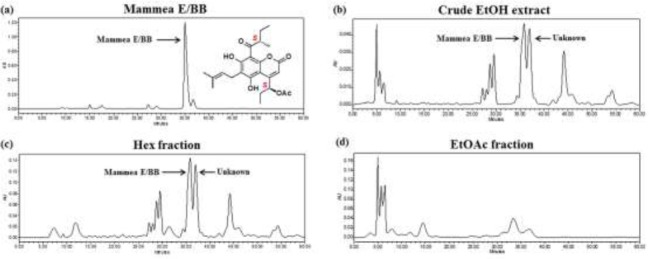
HPLC fingerprints of crude and fractional extracts from flowers of M. siamensis. (a) Standard mammea E/BB, (b) crude EtOH extract, (c) Hex fraction, and (d) EtOAc fraction.
Effect of M. siamensis flower extracts on WT1 protein expression in Molt4 and K562 cell lines
Molt4 and K562 cells were incubated with crude EtOH extract, Hex, and EtOAc fractions with the concentrations at IC20 values for 48 h (Table 1), after which cells were harvested and counted. The WT1 protein levels after the treatments were significantly decreased in Molt4 (Fig. 3a) and K562 cells (Fig. 3b). The most effective extract was the Hex fraction, with the percent inhibition of 68.4 ± 6.4 and 72.1 ± 1.9%, respectively when compared to the vehicle control. Moreover, crude EtOH and EtOAc fraction significantly decreased WT1 protein levels in Molt4 cells, while there was no effect of EtOAc in K562 cells. The total cell numbers after Hex fraction treatments for 48 h in Molt4 and K562 cells were decreased by 66.2 ± 3.4% (Fig 3d) and 48.7 ± 0.4% (Fig 3e), respectively, when compared to the vehicle control.
Figure 3.
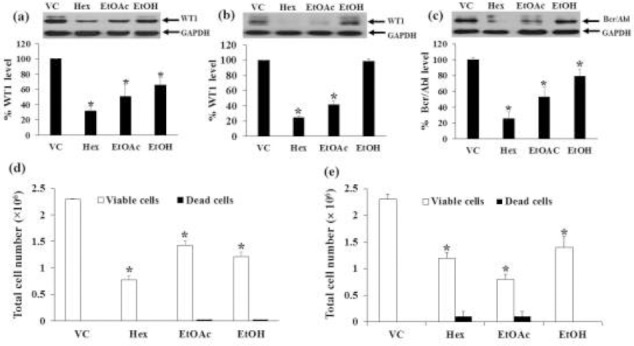
Effects of EtOH extract, Hex and EtOAc fractions on WT1 protein expression in Molt4 and K562 cells and Bcr/Abl in K562 cells. The levels of WT1 protein expression in (a) Molt4 and (b) K562 cells and Bcr/Abl protein expression in (c) K562 cells after treatments were evaluated by Western blotting; GAPDH was used as a loading control. The total cell numbers of (d) Molt4 and (e) K562 cells were determined by the trypan blue dye exclusion method. Data are the mean values ± SEM of three independent experiments. * denotes a significant difference from the control group (p < 0.05).
Effects of M. siamensis flower extracts on Bcr/Abl protein expression in K562 cells
The Bcr/Abl protein levels in K562 cells were decreased after treatments with crude EtOH extract, Hex, and EtOAc fractional extracts with the concentration at IC20 values for 48 h. The Hex fraction was the most effective fraction that inhibited the Bcr/Abl expression (74.6 ± 8.6%) compared to the vehicle control, followed by the EtOAc fraction (46.9 ± 12.2%) (Fig. 3c). The total cell numbers were decreased by 48.7 ± 0.4 and 75.3 ± 1.5%, respectively compared to the vehicle control (Fig. 3e).
Effect of incubation time period of M. siamensis flower extracts on WT1 and Bcr/Abl protein expression and total cell number in leukemic cells
The Molt4 and K562 cells were treated with crude EtOH extract, Hex, and EtOAc fractions for 12, 24, 48, and 72 h. The WT1 protein levels were determined by Western blotting. Crude EtOH, Hex, and EtOAc fractions decreased WT1 protein levels with increasing time in Mol4 cells (Fig. 4a, Fig. 4b, c). However, the Hex and EtOAc fractions suppressed WT1 protein expression more than the crude EtOH. The Hex extract and EtOH treatments decreased WT1 protein levels in K562 cells with increased treatment time (Fig. 5a, Fig. 5c), while the EtOAc fraction did not (Fig. 5b).
Figure 4.
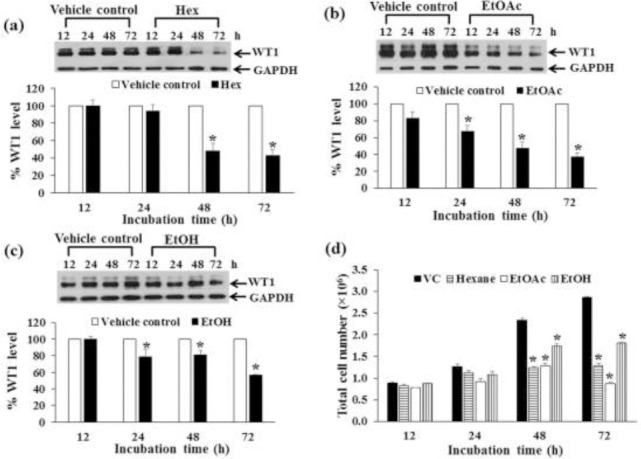
Effect of time period of crude EtOH extract, Hex, and EtOAc fraction treatments on WT1 expression in Molt4 cells. Molt4 cells were treated with crude EtOH extract, Hex fraction, and EtOAc fraction at the concentrations of IC20 values for 12, 24, 48, and 72 h. The levels of WT1 protein expression after being treated with (a) Hex fraction, (b) EtOAc fraction, and (c) crude EtOH extract were evaluated by Western blotting; GAPDH was used as a loading control. (d) The total cell numbers after 12, 24, 48, and 72 h were determined by the trypan blue dye exclusion method. * denotes a significant difference from the control group (p < 0.05).
Figure 5.
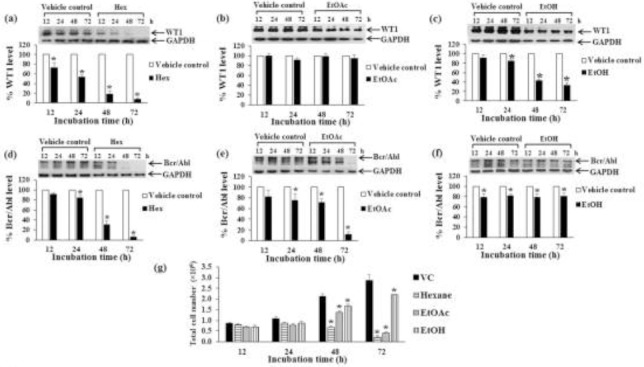
Effect of time periods of crude EtOH extract, Hex, and EtOAc fraction treatments on Bcr/Abl and WT1 protein expression in K562 cell line. K562 cells were treated with Hex fraction (a, d), EtOAc fraction (b, e), and crude EtOH extract (c, f) at the concentrations of IC20 values for 12, 24, 48, and 72 h. The levels of WT1 (a, b, c) and Bcr/Abl (d, e, f) proteins were evaluated by Western blotting; GAPDH was used as a loading control. The levels of WT1 and Bcr/Abl protein expressions were quantified by a scanning densitometer. (g) The total cell numbers after 12, 24, 48, and 72 h were determined by the trypan blue exclusion method. * denotes a significant difference from the control group (p < 0.05).
Bcr/Abl protein expression in K562 cells was examined for treatment time response. Treatments of K562 cells with Hex and EtOAc fractions for 12, 24, 48, and 72 h decreased Bcr/Abl protein levels with increasing time (Fig. 5d, Fig. 5e). Treatment of K562 cells with crude EtOH extract decreased Bcr/Abl protein levels but the effect did not change with increasing time (Fig. 5f).
The total cell numbers of Molt4 and K562 cells after crude EtOH extract, Hex, and EtOAc fractions with the same concentration as above (IC20 value) for 48 h were decreased by a time dependent manner as shown in Fig. 4d and 5g. However, the crude EtOH extract and fractional extracts did not induce dead cells after treatment. The percent of dead cells were in the range of 0-3%.
The active compound of the M. siamensis flower extract was thus most likely found in the low to nonpolar compartment, especially in the Hex fraction. Thus the Hex fraction was selected for further analysis.
Effect of dosage of Hex fraction from M. siamensis flowers on WT1 protein expression in Molt4 and K562 cells
Various doses (non-cytotoxic levels) of the Hex fraction of M. siamensis flowers were examined for their effect on WT1 protein expression. The nontoxic doses of the Hex fraction used for Molt4 cells were 0.5, 1.0, 1.5, and 2.0 μg/ml and for K562 cells were 25, 30, 35, and 40 μg/ml. The Hex fraction significantly decreased WT1 protein levels in Molt4 and K562 cells with increasing doses (Fig. 6a, 7a). The total cell numbers decreased with increasing dosage at 48 h in Molt4 and K562 cells as shown in Fig. 6b and 7c, respectively.
Figure 6.
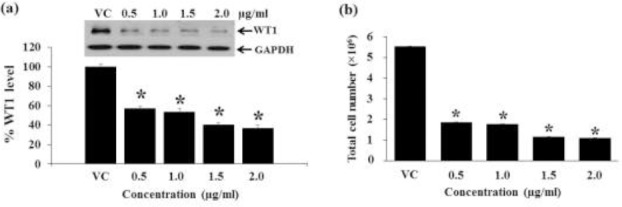
Effect of dosages of the Hex fraction on WT1 protein expression and total cell number in Molt4 cells. Molt4 cells were treated with various doses (0.5, 1.0, 1.5, and 2.0 μg/ml) for 48 h. (a) The levels of WT1 expression after treatment were evaluated by Western blotting; GAPDH was used as a loading control. (b) The total cell numbers were determined by the trypan blue dye exclusion method. * denotes a significant difference from the control group (p < 0.05).
Figure 7.
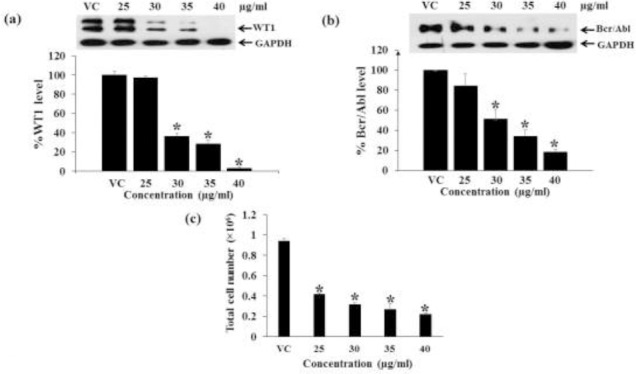
Effect of dosages of the Hex fraction on WT1 and Bcr/Abl expression in K562 cells. K562 cells were treated with various doses (25, 30, 35, and 40 μg/ml) for 48 h. (a) The levels of WT1 protein expression and (b) Bcr/Abl expression after treatments were evaluated by Western blotting; GAPDH was used as a loading control. (c) The total cell numbers were determined by the trypan blue dye exclusion method. * denotes a significant difference from the control group (p < 0.05).
Inhibitory effect of dosage of Hex fraction from M. siamensis flowers on Bcr/Abl protein expression in K562 cells
Various doses (25, 30, 35, and 40 μg/ml) of the Hex fraction of M. siamensis flowers were examined for their effect on Bcr/Abl protein expression in K562 cells. The levels of Bcr/Abl protein were significantly decreased at 48 h when compared to the vehicle control (Fig. 7b).
Effect of mammea E/BB on WT1 and Bcr/Abl protein expressions in Molt4 and K562 cells
To compare the inhibitory effects of the main active compound (mammea E/BB) and active component of the fractional extract (Hex) on WT1and Bcr/Abl proteins, Molt4 and K562 cells were tested for their activity. The Hex fraction and mammea E/BB at concentrations equal to the IC20 values (30.6 and 75.0 μg/ml, respectively) were added to K562 cells for 48 h. In Molt4 cells, the IC20 values for the Hex fraction and mammea E/BB were 1.0 and 2.1 μg/ml, respectively from the MTT assay (Fig. 1a, 8a). The levels of WT1 protein were significantly decreased, by 50.6 ± 3.1 and 75.0 ± 5.6%, in response to the Hex fraction and mammea E/BB treatment respectively, compared to the vehicle control (p < 0.05) (Fig. 8b). The IC20 values for the Hex fraction and mammea E/BB in K562 were 30.6 and 75 μg/ml, respectively (Fig. 1b, 8d). The protein levels of Bcr/Abl were significantly decreased by 72.5 ± 8.4 and 49.5 ± 4.0%, in response to the Hex fraction and mammea E/BB treatment respectively, compared to the vehicle control (p < 0.05) (Fig. 8e). The total cell numbers after Hex fraction and mammea E/BB treatments in Molt4 were significantly decreased by 29.5 ± 3.4 and 63.6 ± 1.5%, respectively (Fig. 8c) and in K562 cells were significantly decreased by 43.9 ± 0.9 and 74.8 ± 1.8%, respectively (Fig. 8f).
Figure 8.
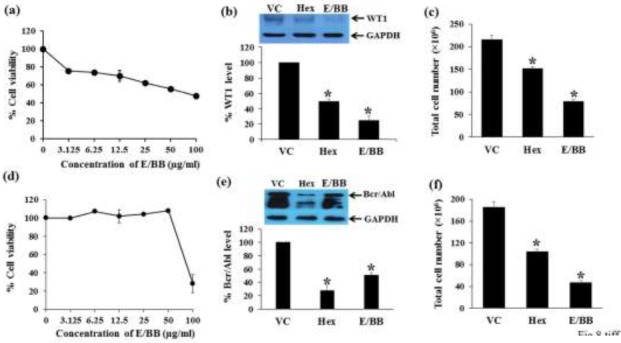
Effect of standard mammea E/BB on cell cytotoxicity and WT1 as well as Bcr/Abl protein expression in Molt4 and K562 cells. (a) Molt4 and (d) K562 cells (1×105 cells/ml) were cultured in the presence of various concentrations of standard mammea E/BB from M. siamensis seeds for 48 h. The cell viability was determined by the MTT assay. The levels of WT1 protein expression after 1.0 μg/ml Hex fraction and 2.1 μg/ml mammea E/BB treatments in Molt4 cells and Bcr/Abl protein expression after 30.6 μg/ml Hex fraction and 75.0 μg/ml mammea E/BB treatments in K562 cells were assessed by Western blotting; GAPDH was used as the loading control. (b) WT1 and (e) Bcr/Abl protein levels in Molt4 and K562 cells, respectively, were analyzed with a scan densitometer. The total cell numbers after Hex fraction and mammea E/BB treatments in (c) Molt4 and (f) K562 cells were determined by the trypan blue dye exclusion method. Data are the mean ± SEM of three independent experiments. * denotes a significant difference from the control group (p < 0.05).
Discussion
The flower of M. siamensis was found to be useful as a heart tonic, a fever-lowering medicine, and for enhancement of appetite in Thai folk medicine. This is the first report of the effect of M. siamensis flower extracts on WT1 and Bcr/Abl proteins in leukemic cells. In this study, the flower crude extract (EtOH) and fractional extracts (Hex, EtOAC, and MeOH) were analyzed for their ability to inhibit leukemic cell growth under suppression of the proteins, WT1 and Bcr/Abl which are involved in human leukemic cell proliferation. The K562 and Molt4 cells were selected to furnish the analysis via the WT1 overexpressing cell model (Anuchapreeda et. al., 2008). Additionally, K562 cells were used for the Bcr/Abl overexpressing cell model (McGahon et. al., 1997). The cytotoxic effects of the Hex fraction were in line with those of the crude EtOH extract which contained both polar and nonpolar organic compounds. Thus the nonpolar Hex fraction was more cytotoxic than the polar MeOH fraction. We thus conclude that the active compounds of the flower extract were nonpolar compounds. The Hex fraction of M. siamensis flowers significantly inhibited cell proliferation via the suppression of WT1 expression in Molt4 and K562 cells and Bcr/Abl expression in K562 cells and decreased total cell numbers. The active compound (s) may be mammea E/BB and/or the unknown compound identified in the HPLC analysis. The mammea E/BB obtained from the n-hexane fraction of a M. siamensis seed extract has been reported to inhibit WT1 protein expression in K562 cells (Rungrojskul et al 2016). However, the n-hexane fraction of M. siamensis seed extract and flower extracts show differences in the HPLC fingerprints. The M. siamensis flower extract from n- hexane contained a higher peak of an unknown compound at the retention time of 37 min.
This current study is the first report of potential biological effects of M. siamensis flowers as used in Thai traditional medicine. Previous chemical studies showed that the flowers of M. siamensis contain several coumarins and xanthones, some of which possess potential biological and therapeutic properties (Kaweetripob et. al., 2000; Ngo et. al., 2010). Recent studies have shown that M. siamensis is a source of mammea E/BB which can inhibit the WT1 protein expression and growth in leukemic cells (Rungrojsakul et. al., 2016). Furthermore, the isolated compounds from of M. siamensis’s flower showed an antiproliferative effects through apoptosis induction in leukemic cells (Tung et. al., 2013). However, there are no previous scientific data to support the effects of M. siamensis flower extract on protein expression in leukemic cells.
Our results demonstrate that the Hex fraction of M. siamensis flowers significantly inhibits cell proliferation via the suppression of WT1 expression in Molt4 and K562 cells and Bcr/Abl expression in K562 cells and decreases total cell numbers. The active compounds may be mammea E/BB or an unknown compound after identified in the HPLC analysis. The known component mammea E/BB was thus examined and its activity compared to that of the Hex fraction. The Hex fraction from M. siamensis flowers suppressed Bcr/Abl and WT1 protein expression and inhibited cell proliferation in leukemic cell lines. The main result in this study agrees with the previous study, the hexane fraction from M. siamensis seed extracts on WT1 protein expression and cell proliferation in the K562 cell line (Rungrojsakul et. al., 2016). The main active compound in the n-hexane extract from seeds of M. siamensis (HSS) was mammea E/BB. WT1 protein expression after mammea E/BB treatment was decreased which was similar to the effect of the HSS. Moreover, pure E/BB and HSS significantly decreased the total cell number at 72 h. (Rungrojsakul et. al., 2016).
The effective compounds in the Hex fraction of M. siamensis flowers need further study. It was suggested that the active compound dissolve in n-hexane, a non-polar solvent, and thus may be an essential oil. The essential oils in M. siamensis flowers include many separate compounds including, mammea B/AC cyclo D, kayeassamin A, surangin C, and theraphin B (Kaweetripob et. al., 2000; Ngo et. al., 2010; Win et. al., 2008).
This study is the first to show that M. siamensis flower extracts have inhibitory effects on Bcr/Abl and WT1proteins in leukemic cells. These results suggest that the hexane extracts of M. siamensis flowers have the ability to inhibit various target proteins related to leukemic cell proliferation, and thus can potentially be used to develop new anti-cancer drugs.
Conclusion
The flower extracts of M. siamensis strongly suppressed the proliferation of leukemia cell lines although the MeOH fraction had neither cytotoxic and nor inhibitory effects on leukemic cells. The Hex fraction decreased the Bcr/Abl and WT1 protein levels, with the effect increasing with dosage and treatment time. This is the first report of the inhibitory effects of M. siamensis flower extracts on Bcr/Abl protein expression in leukemic cells. The active compounds are certainly nonpolar. The main peaks of the Hex fraction correspond to mammea E/BB and an unknown compound. Moreover, mammea E/BB displayed inhibitory effects on both Bcr/Abl and WT1protein expression. However, the unknown compound should be further investigated its activity and compared to that of mammea E/BB. Our results indicate that flower extracts from M. siamensis may be useful alternative therapeutic agents in human leukemias. This study has provided a basis for the future development of M. siamensis as an alternative natural leukemia treatment.
Acknowledgements
The authors are grateful for grants received from the Thailand Research Fund (TRF), Grant number RSA5580029, and the National Research Council (NRC) and Chiang Mai University Research Fund under Grant number 2558A10401001.
Author disclosure statement
No competing financial interests exist.
References
- 1.Anuchapreeda S, Tima S, Duangrat C, Limtrakul P. Effect of pure curcumin, demethoxycurcumin, and bisdemethoxycurcumin on WT1 gene expression in leukemic cell lines. Cancer Chemother. Pharmacol. 2008;62:585–594. doi: 10.1007/s00280-007-0642-1. [DOI] [PubMed] [Google Scholar]
- 2.Inoue K, Sugiyama H, Ogawa H, Nakagawa M, Yamagami T, Miwa H, Kita K, Hiraoka A, Masaoka T, Nasu K, Kyo T, Dohy H, Nakauchi H, Ishidate T, Akiyama T, Kishimoto T. WT1 as a new prognostic factor and a new marker for the detection of minimal residual disease in acute leukemia. Blood. 1994;84:3071–3079. [PubMed] [Google Scholar]
- 3.Kaweetripob W, Mahidol C, Prawat H, Ruchirawat S. Chemical investigation of mammea siamensis. Pharm. Biol. 2000;38(Suppl 1):55–57. doi: 10.1076/phbi.38.6.55.5954. [DOI] [PubMed] [Google Scholar]
- 4.McGahon AJ, Brown DG, Martin SJ, Amarante-Mendes GP, Cotter TG, Cohen GM, Green DR. Downregulation of Bcr-Abl in K562 cells restores susceptibility to apoptosis: characterization of the apoptotic death. Cell Death Differ. 1997;4:95–104. doi: 10.1038/sj.cdd.4400213. [DOI] [PubMed] [Google Scholar]
- 5.Menke AL, van der Eb AJ, Jochemsen AG. The Wilms’ tumor 1 gene: oncogene or tumor suppressor gene? Int. Rev. Cytol. 1998;181:151–212. doi: 10.1016/s0074-7696(08)60418-0. [DOI] [PubMed] [Google Scholar]
- 6.Ngo NT, Nguyen VT, Vo HV, Vang O, Duus F, Ho TD, Pham HD, Nguyen LH. Cytotoxic Coumarins from the Bark of Mammea siamensis. Chem. Pharm. Bull. (Tokyo) 2010;58:1487–1491. doi: 10.1248/cpb.58.1487. [DOI] [PubMed] [Google Scholar]
- 7.Perez-Caro M, Sanchez-Garcia I. BCR-ABL, Human Cancer. In: Srivastava R, editor. Apoptosis, Cell Signaling, and Human Diseases. Humana Press; 2007. pp. 3–34. [Google Scholar]
- 8.Poobrasert O, Constant HL, Beecher CW, Farnsworth NR, Kinghorn AD, Pezzuto JM, Cordell GA, Santisuk T, Reutrakul V. Xanthones from the twigs of Mammea siamensis. Phytochemistry. 1998;47:1661–1663. doi: 10.1016/s0031-9422(97)00820-0. [DOI] [PubMed] [Google Scholar]
- 9.Rungrojsakul M, Saiai A, Ampasavate C, Anuchapreeda S, Okonogi S. Inhibitory effect of mammea E/BB from Mammea siamensis seed extract on Wilms’ tumour 1 protein expression in a K562 leukaemic cell line. Nat. Prod. Res. 2016;30:443–447. doi: 10.1080/14786419.2015.1017491. [DOI] [PubMed] [Google Scholar]
- 10.Tamaki H, Ogawa H, Inoue K, Soma T, Yamagami T, Miyake S, Oka Y, Oji Y, Tatekawa T, Tsuboi A, Tagawa S, Kitani T, Aozasa K, Kishimoto T, Sugiyama H, Miwa H, Kita K. Increased expression of the Wilms tumor gene (WT1) at relapse in acute leukemia. Blood. 1996;88:4396–4398. [PubMed] [Google Scholar]
- 11.Tung NH, Uto T, Sakamoto A, Hayashida Y, Hidaka Y, Morinaga O, Lhieochaiphant S, Shoyama Y. Antiproliferative and apoptotic effects of compounds from the flower of Mammea siamensis (Miq.) T, ers. on human cancer cell lines. Bioorg. Med. Chem. Lett. 2013;23:158–162. doi: 10.1016/j.bmcl.2012.10.127. [DOI] [PubMed] [Google Scholar]
- 12.Win NN, Awale S, Esumi H, Tezuka Y, Kadota S. Novel anticancer agents, kayeassamins A and B from \the flower of Kayea assamica of Myanmar. Bioorg. Med. Chem. Lett. 2008;18:4688–4691. doi: 10.1016/j.bmcl.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Yamagami T, Sugiyama H, Inoue K, Ogawa H, Tatekawa T, Hirata M, Kudoh T, Akiyama T, Murakami A, Maekawa T. Growth inhibition of human leukemic cells by WT1 (Wilms tumor gene) antisense oligodeoxynucleotides: implications for the involvement of WT1 in leukemogenesis. Blood. 1996;87:2878–2884. [PubMed] [Google Scholar]
- 14.Yang L, Han Y, Suarez Saiz F, Minden MD. A tumor suppressor and oncogene: the WT1 story. Leukemia. 2007;21:868–876. doi: 10.1038/sj.leu.2404624. [DOI] [PubMed] [Google Scholar]


