Abstract
Glutamate dehydrogenase (GDH) produces a precursor to glutathione, an important molecule in maintaining cellular redox balance and the cancerous characteristics of tumor cells through intracellular signaling pathways. However, the underlying molecular mechanisms linking glutamate dehydrogenase and extrahepatic cholangiocarcinoma have not been elucidated yet. Herein, we examined GDH expression levels and evaluated its potential correlations with prognosis. Meanwhile, the therapeutic value of GDH targeting the Smad pathways in extrahepatic cholangiocarcinoma was explored. Immunohistochemical studies revealed that GDH expression level was correlated to CD34 expression, cellular differentiation, the presence or absence of capsular and vascular invasion, lymph node metastasis, neural invasion and patient age. Kaplan-Meier survival analysis and COX proportional hazards models demonstrated that the prognosis was closely associated with GDH expression, CD34 positivity, nerve infiltration and cell differentiation. GDH silencing significantly reduced the proliferation, migratory potential and invasive capability. We also demonstrated that GDH promoted cell proliferation and metastasis potentially through Smad-mediated induction of TGF-β signaling pathway. Therefore, GDH may be an important prognostic indicator and may provide a new target for novel treatments of extrahepatic cholangiocarcinoma.
Keywords: Extrahepatic cholangiocarcinoma, prognosis, GDH, Smad signaling pathway, TGF-β
Introduction
The prognosis of cholangiocarcinoma is historically poor since that early diagnosis is difficult. Insidious onset and sub-clinical symptoms result in a low survival rate. Progression of extrahepatic cholangiocarcinoma may be related to interactions between susceptibility genes and the environmental, however, extrahepatic cholangiocarcinoma has high morbidity with its pathogenesis not well understood [1,2]. Currently, surgery is the only curative treatment. Recent research has demonstrated that the combination of new adjuvant chemotherapy with radical cholangiocarcinoma surgery improves patient long-term survival [3]. For unresectable extrahepatic cholangiocarcinoma, chemotherapy and radiotherapy have no definitive therapeutic effect. To date, there is no sufficient evidence demonstrating chemotherapy can significantly improve survival in extrahepatic cholangiocarcinoma patients with or without the surgical resection [4]. Most patients whose tumors cannot be resected die within 12 months of diagnosis due to cachexia and deteriorating physical condition [5]. Liver failure, severe sepsis and secondary obstructive jaundice accelerate the death rate. Less than 5% of extrahepatic cholangiocarcinoma patients survive more than five years, and the survival rate has not improved significantly in the past 30 years [6].
The current study focused on signaling pathways underpinning extrahepatic cholangiocarcinoma development. The transforming growth factor-β (TGF-β) signaling pathway plays a complex role in embryonic development and physiological and pathological development in adults, showing a popular target in cancer researches [7,8]. The TGF-β superfamily is responsible for a variety of biological functions, including regulation of cell proliferation, differentiation, migration, survival, angiogenesis and immune monitoring [9]. The receptors for the TGF-β superfamily are transmembrane serine/threonine kinase receptors that are distributed on the cell membrane and are divided into type I and type II. Activation of serine/threonine kinase type I receptors may trigger many downstream signaling pathways [10], including the Smad signaling pathway, which comprises elements termed Smads that play a key role in the TGF-β signaling pathway. Aberrant expression or dysfunction of Smad can significantly alter the effects of the TGF-β pathway.
During tumorigenesis and development, a series of changes occur both inside and outside tumor cells. An important feature of cancer cells is a change in cellular energy metabolism. To maintain rapid growth during cancer cell proliferation, cancer cells must accelerate the synthesis of ATP, nucleotides, lipids and other biological macromolecules; only with adequate nutrition can normal cell division be ensured [11]. Although the relationship between cellular metabolism and tumorigenesis was first addressed nearly 100 years ago by Otto Warburg, the molecular mechanisms linking metabolic pathways and tumorigenesis have been revealed only recently, introducing a new area of cancer research.
Glutamate dehydrogenase (GDH) plays a key role in glutamine metabolism. Glutamine metabolism and its role in tumor cells is of considerable interest [12]. Glutamine metabolism provides energy and the material basis for rapid tumor cell growth and proliferation; GDH plays a key role in the generation of glutathione, which is an important molecule in maintaining cellular redox balance. GDH also plays a role in maintaining the cancerous characteristics of tumor cells by influencing intracellular signaling pathways [13]. Glutamine metabolism also plays an important role in mTORC 1 activity [14]. Thus, inhibition of glutamine metabolism can provide an effective strategy for cancer therapy. However, the expression and prognostic value of GDH in extrahepatic cholangiocarcinoma has not been well investigated.
In the present study, we examined the expression of GDH in extrahepatic cholangiocarcinoma and analyzed the clinical significance of GDH in a cohort of extrahepatic cholangiocarcinoma patients. In addition, we explored the potential role of GDH in the proliferation and motility of extrahepatic cholangiocarcinoma cells. Our results provide insights into the pathogenesis of extrahepatic cholangiocarcinoma and may provide a novel therapeutic target for the treatment of extrahepatic cholangiocarcinoma.
Material and methods
Tissue sample collection
We collected 155 paraffin-embedded biopsy specimens from patients with extrahepatic cholangiocarcinoma at Sun Yat-sen Memorial Hospital from June 2005 to June 2015. All the patients were pathologically diagnosed with extrahepatic cholangiocarcinoma after surgery without receiving chemotherapy or radiotherapy. The cohort included 103 males and 52 females aged 37 to 79 years old (median age of 63 years), with 66 cases <60 years old and 89 cases ≥60 years old. Fifty samples were CD34+, and 105 samples were CD34-. According to the WHO pathological classification criteria, 58 cases were highly differentiated adenocarcinomas, 49 cases were moderately differentiated adenocarcinomas, and 48 cases were poorly differentiated adenocarcinomas. Visual and/or pathological analysis identified vascular thrombosis in 24 cases; vascular invasion in 131 cases; lymph node metastases in 46 cases; no lymph node metastases in 109 cases; enveloped and neural invasion in 50 cases; and no neural invasion in 105 cases. In addition, we collected fresh frozen specimens of surgically removed extrahepatic tissues for nearly two years from our hospital; adjacent normal biliary mucosal tissues were collected in 32 cases for subsequent experiments.
Immunohistochemistry
The immunohistochemical SP staining technique was used to detect the protein expression of GDH in extrahepatic cholangiocarcinoma samples/cells. A standard extrahepatic cholangiocarcinoma specimen was used as a positive control and the primary antibody was replaced by PBS as a negative control; 11 normal bile duct tissues were used as normal tissue controls. After screening according to the above conditions, a final total of 155 cases of patients conformed to the inclusion criteria. In this study, tumor differentiation was characterized as low, medium or high grade.
A standard for positive result determination: The staining intensity of GDH-positive samples ranged from light yellow to tan to sepia and was mainly located in the cytoplasm. We scored positive cells using a two-stage method based on the proportion of positive cells in each of five representative high-power fields using a Leica DMR microscope (Leica Microsystems, Wetzlar, Germany) (0. no positive cells; 1. positive cells less than 10%; 2. positive cells between 10% and 35%; 3. positive cells between 35% and 70%; 4. positive cells more than 70%). The staining intensity was classified as follows: light yellow received 1 point, yellow or dark yellow received 2 points, and brown or tan received 3 points. The total score was obtained by multiplying the two individual scores, with results ranging from 0 (no positive cells) to 12 (a score of 4 with strong positive expression). To facilitate comparison among lesions with different staining intensities, the results of the above scoring were differentiated into three categories: + <75, ++ 75-150, +++ >150. In the double-blind method, each slice was read by two people, and re-analysis was performed if the counts differed by >10% (Table 1).
Table 1.
Distribution of immunohistochemical staining intensity in 156 patients withcholangiocarcinoma
| Parameters | Cases | GDH staining intensity | P | |||
|---|---|---|---|---|---|---|
|
| ||||||
| + | ++ | +++ | ||||
| CD34 | + | 50 | 2 (4.0%) | 16 (32.0%) | 32 (64.0%) | 0.000 |
| - | 105 | 38 (36.2%) | 41 (39.0%) | 26 (24.8%) | ||
| Gender | Male | 102 | 25 (24.5%) | 37 (36.3%) | 40 (39.2%) | 0.790 |
| Femal | 53 | 15 (28.3%) | 20 (37.7%) | 18 (34.0%) | ||
| Differentiation | High | 58 | 39 (67.2%) | 18 (31.%) | 1 (1.7%) | 0.000 |
| Middle | 49 | 1 (2.0%) | 37 (75.5%) | 11 (22.4%) | ||
| Low | 48 | 0 (0.0%) | 2 (4.2%) | 46 (95.8%) | ||
| Capsular invasion | + | 105 | 23 (21.9%) | 39 (37.1%) | 43 (41.0%) | 0.221 |
| - | 50 | 17 (34.0%) | 18 (36.0%) | 15 (30.0%) | ||
| Vascular invasion | + | 24 | 1 (4.2%) | 9 (37.5%) | 14 (58.3%) | 0.014 |
| 131 | 39 (29.8%) | 48 (36.6%) | 44 (33.6%) | |||
| Lymph node | + | 46 | 6 (13.0%) | 20 (43.5%) | 20 (43.5%) | 0.062 |
| - | 109 | 34 (31.2%) | 37 (339%) | 38 (34.9%) | ||
| Neural invasion | + | 50 | 3 (6.0%) | 17 (34.0%) | 30 (60.0%) | 0.000 |
| - | 105 | 37 (35.2%) | 40 (38.1%) | 28 (26.7%) | ||
| Age* | <40 | 2 | 1 (50.0%) | 0 (0.0%) | 1 (50.0%) | 0.000 |
| 40-49 | 18 | 8 (44.4%) | 5 (27.8%) | 5 (27.8%) | ||
| 50-59 | 41 | 24 (58.5%) | 6 (14.6%) | 11 (26.8%) | ||
| 60-69 | 59 | 6 (10.2%) | 35 (59.3%) | 18 (30.5%) | ||
| ≥70 | 35 | 1 (2.9%) | 11 (31.4%) | 23 (65.7%) | ||
Nonparametric test.
Regular follow-up
All patients underwent standardized follow-up. The follow-up examination included abdominal ultrasound, chest X-ray, liver function tests, serum CA19-9 and CEA levels. Patients were examined once every two months for two years after surgery, then every three months thereafter. Contrast-enhanced CT or MRI was performed at six-month intervals after surgery. The study end point was June 31, 2015. The preoperative diagnostic criteria were consistent with the tumor recurrence diagnostic criteria, including ultrasound, contrast-enhanced CT, MRI or hepatic artery angiography.
Cell culture and subculture
Human extrahepatic cholangiocarcinoma cell lines (QBC, RBE, FRN, FRH0201, 9810 and HuH-28) were purchased from the Gisele biotechnology company (Guangzhou, China). Cells were cultured in RPMI 1640 medium supplemented with 20% FBS; cells were maintained at 37°C in an incubator with 5% CO2. Cells were were grown to ~80-90% confluency and passaged after conventional trypsinization. Briefly, the medium was aspirated from the flask, and the cells were rinsed twice with PBS. Then, 0.25% trypsin was added, and the cells were observed under an inverted microscope until the cells became rounded with a larger diopter value. After an increase in the cell gap was observed, trypsin was removed, fresh medium was added, and the cells were gently dispersed by pipetting and seeded in a new flask. The GDH short hairpin RNA (shRNA) was synthesized and cloned into a pLKO.1-TRC vector (Addgene plasmid # 10878). Vectors were co-transfected into 293T cells along with the retroviral packaging plasmid. Following transfection, supernatants were harvested and used to infect extrahepatic cholangiocarcinoma cells, and the stably transfected cells were selected with puromycin according to the manufacturer’s instructions.
Detection of mRNA expression level before and after GDH silencing in extrahepatic cholangiocarcinoma cells and tissues by qRT-PCR
Bile duct cancer cells were seeded onto 6-well plates and cultured for 24 hrs. The cells were then harvested, and total RNA was extracted using TRIzol and reverse transcribed to cDNA; 18S RNA primers were used as an internal control. Standard curves were generated for GDH and 18S RNA and used for relative quantification. Sequences were generated using DNASIS software, and homology analysis was performed using CLUSTALW software; sequencing results were compared and phylogenetic analysis was performed using MEGA software. The primers for qRT-PCR were as follows GDH: Forward: 5’-GGG ATT CTA ACT ACC ACT TGC TCA-3’, Reverse: 5’-AAC TCT GCC GTG GGT ACA AT-3’; GAPDH: Forward: 5’-CTC CTC CTG TTC GAC AGT CAG C-3’, Reverse: 5’-CCC AAT ACG ACC AAA TCC GTT-3’. Groups were analyzed in triplicate, and the results were averaged.
Detection of GDH protein levels before and after transfection of extrahepatic cholangiocarcinoma cells and tissues by Western blotting
Total protein was extracted from bile duct cancer cell lines before and after GDH silencing. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal reference, and the protein concentration was determined using the bicinchoninic acid (BCA) method. A 30 μg quantity of total protein was separated on a polypropylene polyacrylamide gel by electrophoresis (SDS-PAGE), followed by transfer to low temperature constant transfer film at 100 V for 1 hr 30 min. The film was incubated with the primary antibody for two hours at room temperature, followed by secondary antibody. Protein bands were detected using an ECL fluorescent color system and quantitatively analyzed using Quantity One software. Groups were analyzed in triplicate, and the results were averaged.
Detection of cell apoptosis before and after transfection of extrahepatic cholangiocarcinoma cells by flow cytometry
Extrahepatic cholangiocarcinoma cells were seeded onto 6-well plates. Cells were transfected for 24 hrs with GDH siRNA or 48 hrs with control siRNA. Following transfection, the cells were treated with 0.25% trypsin, 1 mM EDTA buffer and fixed in 70% ethanol at 4°C. Cellular apoptosis was analyzed by flow cytometry using the Annexin V apoptosis detection kit according to the manufacturer’s instructions.
Detection of cell proliferation before and after transfection of extrahepatic cholangiocarcinoma cells by MTT assay
At 24 hrs after transfection, the cells were digested with 0.25% trypsin, centrifuged at 1000 rpm for 10 min, and resuspended in RPMI 1640 medium supplemented with 10% fetal bovine serum. Paired single-cell suspensions from the GDH and control siRNA groups were prepared as 1 × 103/well in 96-well plates and cultured at 37°C and 5% CO2. Cells from the two groups (control siRNA transfection and experimental GDH siRNA transfection groups) were cultured for 96 hrs; 100 µl of MTT (5 mg/ml) was then added, and the cells were incubated for an additional 4 hrs. Next, 100 µl of DMSO was added to each well, and the cells were incubated until optical microscopy indicated that the formazan was completely dissolved. The absorbance was then measured at 570 nm. Empty wells and wells containing only culture medium were used as controls.
Detection of cell migration before and after GDH silencing in extrahepatic cholangiocarcinoma cells by the Scratch test
Cell culture and transfection were performed as described above. At 24 h after transfection, cells were digested with 0.25% trypsin, centrifuged at 1000 rpm for 10 min, and resuspended in RPMI 1640 medium supplemented with 10% fetal bovine serum. Cells were seeded as single-cell suspensions of 1 × 105/ml, 1 ml into 6-well plates or as whole-cell monolayers cultured in serum-containing medium. Adherent cells in the medium were aspirated with a micropipette-like tip to create a 1-mm cell-free zone that was washed with serum-free medium or PBS to remove any remaining cells. The cell-free area was marked, serum-containing medium was added, and the cells were returned to the incubator for 24 to 48 hrs. Images were acquired at different time points during the incubation.
Detection of the invasive ability of extrahepatic cholangiocarcinoma cells before and after GDH silencing by Transwell assay
Cell culture and transfection were performed as described above. At 24 hrs after transfection, cells were digested with 0.25% trypsin, centrifuged at 1000 rpm for 10 min, and resuspended in RPMI 1640 medium supplemented with 10% fetal bovine serum. Cells were seeded as single-cell suspensions of 1 × 105 cells/well in Transwell invasion chambers with 700 µl of RPMI 1640 medium supplemented with 10% fetal bovine solution. Cells from the two groups (empty vector control group and experimental group) were incubated for 48 hrs at 37°C with 5% CO2. Cells were fixed in a neutral formalin solution and examined on an inverted microscope at low magnification; three fields were chosen at random, and the number of cells was counted under high magnification to determine the mean number of cells per field. Experiments were repeated in triplicate.
Statistical analysis
Data are presented as the mean ± SD, and differences between groups were analyzed using Student’s t-test, the chi-squared test or the fisher exact test. The Kaplan-Meier method and log-rank test were used to estimate survival rates. The Cox proportional hazards model was used to calculate univariate and multivariate hazard ratios for the study variables. Statistical analyses were performed with SPSS 20.0 software (Chicago, IL, USA), and results with P<0.05 were considered statistically significant.
Results
GDH expression in extrahepatic cholangiocarcinoma tissues
Immunohistochemical analysis of paraffin-embedded tissue sections revealed that GDH expression was primarily located in the cytoplasm and cell membrane of extrahepatic cholangiocarcinoma cells, with some nuclear expression. The staining intensity ranged from light yellow to tan to sepia (Figure 1). In extrahepatic cholangiocarcinoma tissues, GDH staining had a patchy or diffuse distribution, while GDH staining in normal bile duct mucosa was scattered. GDH expression was increased in extrahepatic cholangiocarcinoma tissue compared with normal bile duct tissue. GDH expression intensity was closely associated with CD34 positivity rate, cell differentiation degree, capsular and vascular invasion depth, lymph node metastasis, neural invasion and patient age.
Figure 1.
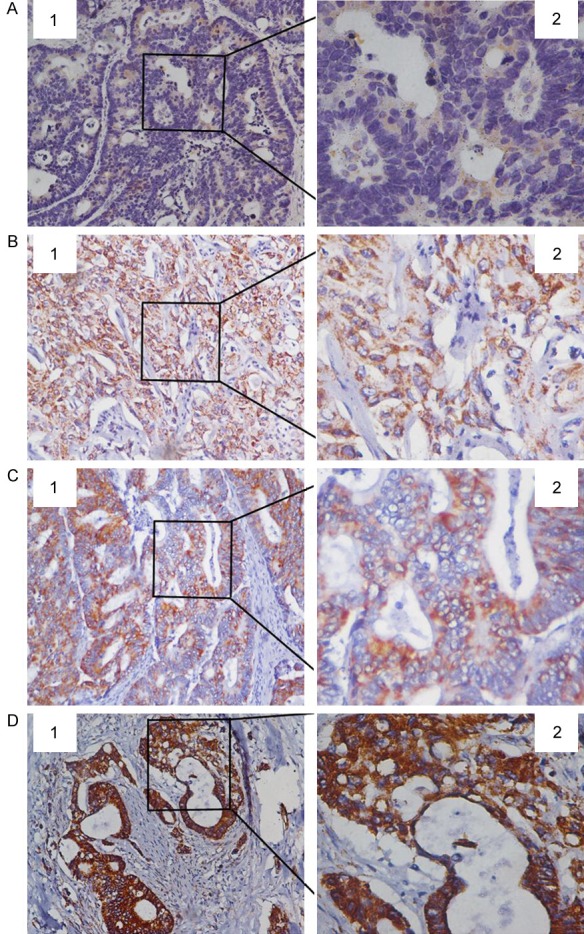
Immunohistochemical staining of GDH expression in para-carcinoma and carcinoma tissues. A: GDH low expression in para-carcinoma tissue; B: GDH low expression in carcinoma tissue; C: GDH middle expression in carcinoma tissue; D: GDH high expression in carcinoma tissue. 1. 20 × magnification; 2. 40 × magnification.
GDH expression level as a prognostic indicator
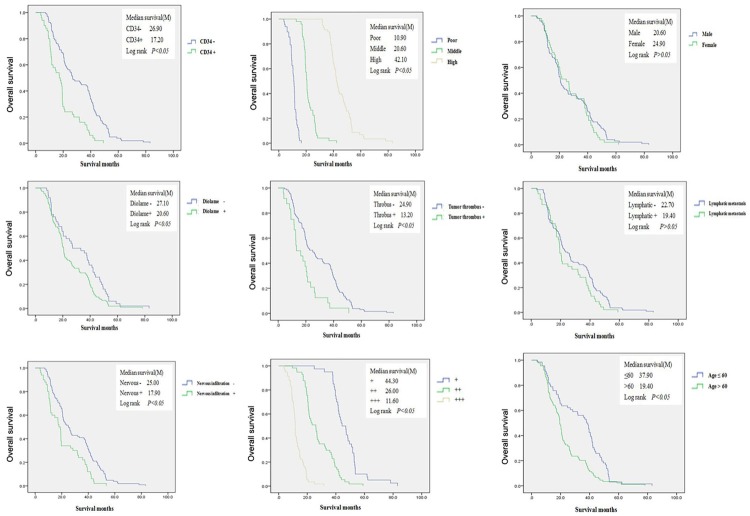
Kaplan-Meier survival curves and log-rank test analyses revealed that the median survival was 44.30 months in patients with low GDH expression [95% CI: 43.795-50.525]; 26.00 months in patients with moderate GDH expression [95% CI: 25.036-30.564]; and 11.60 months in patients with high GDH expression [95% CI: 11.314-13.948]. Single-factor and multi-factor survival analyses indicated that the prognosis of extrahepatic cholangiocarcinoma patients was closely associated with GDH expression (HR 3.80; 95% CI: 1.51-9.90; P=0.005), CD34 positivity (HR 2.02; 95% CI: 1.36-3.01; P=0.001), nerve infiltration (HR 2.55; 95% CI: 1.58-4.11; P=0.000) and cell differentiation (HR 0.001 95% CI: 0.000-0.006 P=0.000, HR 0.12 95% CI: 0.003-0.059; P=0.000) (Figure 2, Table 2).
Figure 2.
Overall survival between groups. Single-factor and multi-factor survival analyses indicated that the prognosis of extrahepatic cholangiocarcinoma patients is closely associated with GDH expression, CD34 positivity, nerve infiltration and cell differentiation.
Table 2.
Results of multivariable Cox proportional hazards model analysis
| Parameters | B | SE | Wald | P | HR (95% CI) |
|---|---|---|---|---|---|
| Staining intensity | |||||
| + | Comparison | ||||
| ++ | 0.531 | 0.305 | 3.031 | 0.082 | 1.70 (0.94-3.09) |
| +++ | 1.354 | 0.479 | 7.958 | 0.005 | 3.8 (1.51-9.90) |
| CD34 (+) | 0.704 | 0.203 | 12.00 | 0.001 | 2.02 (1.36-3.01) |
| Neural invasion | 0.936 | 0.244 | 14.754 | 0.000 | 2.55 (1.58-4.11) |
| Differentiation | |||||
| Low | Comparison | ||||
| Middle | -4.392 | 0.799 | 30.193 | 0.000 | 0.12 (0.003-0.059) |
| High | -6.911 | 0.872 | 62.860 | 0.000 | 0.001 (0.000-0.006) |
Impact of GDH silencing on the proliferation, migration and invasive capacity of extrahepatic cholangiocarcinoma cells
Scratch tests demonstrated that silencing GDH significantly decreased the migratory ability of extrahepatic cholangiocarcinoma cells compared with the control group (Figure 3). MTT assays of GDH-silenced and control cells revealed that silencing GDH significantly inhibited cell proliferation and the growth of adherent cells compared with the control group (Figure 4). Transwell assays demonstrated that silencing GDH significantly decreased the invasive potential of extrahepatic cholangiocarcinoma cells (Figure 4).
Figure 3.
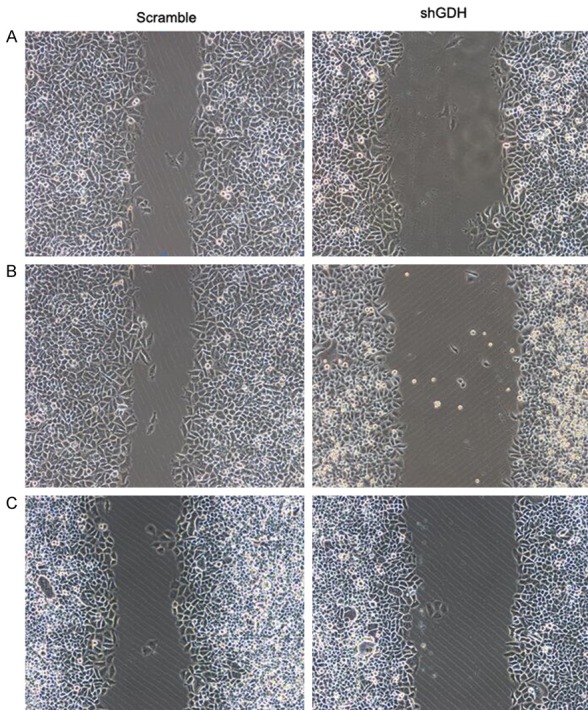
Cell migration before and after GDH silencing in different extrahepatic cholangiocarcinoma cells. A: QBC extrahepatic cholangiocarcinoma cells; B: RBE extrahepatic cholangiocarcinoma cells; C: FRN extrahepatic cholangiocarcinoma cells.
Figure 4.
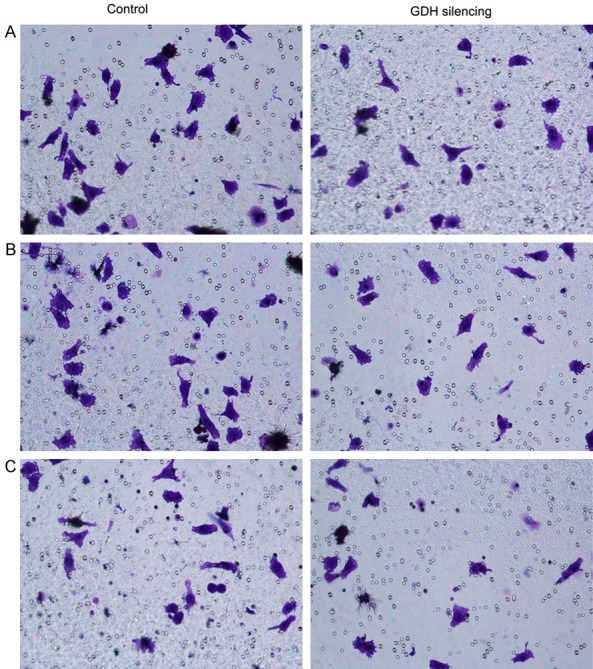
Cell invasive capacity before and after GDH silencing in extrahepatic cholangiocarcinoma cells. A: QBC extrahepatic cholangiocarcinoma cells; B: RBE extrahepatic cholangiocarcinoma cells; C: FRN extrahepatic cholangiocarcinoma cells.
Cell apoptosis after GDH silencing in extrahepatic cholangiocarcinoma cells
GDH silencing enduced a significantly higher rate of apoptosis of extrahepatic cholangiocarcinoma cells, as determined by flow cytometry (Figure 5).
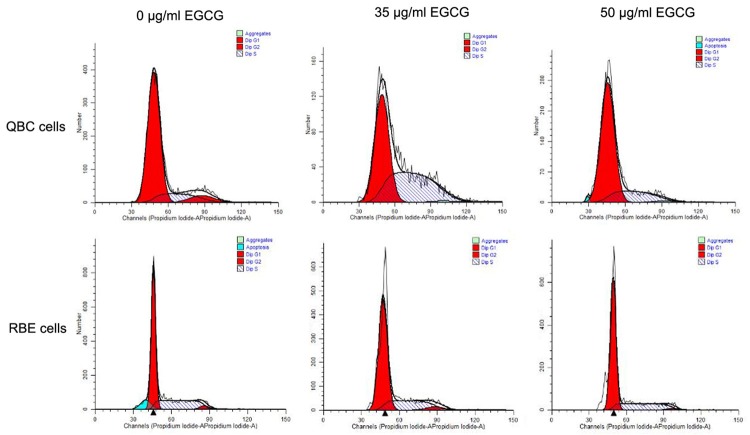
Figure 5.
The effect of EGCG on the cell cycle of extrahepatic cholangiocarcinoma cells. Abbreviation: EGCG, epigallocatechin gallate.
mRNA and protein expression after GDH silencing in cholangiocarcinoma cells and tissues
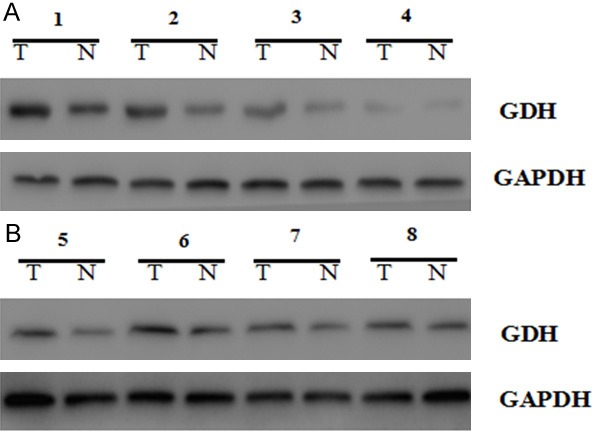
GDH mRNA expression levels were detected by qRT-PCR in extrahepatic cholangiocarcinoma cells and tissues. GDH silencing significantly decreased GDH mRNA expression levels (Figure 6, P<0.05). Western blot results showed that GDH protein expression level decreased significantly after GDH silencing in extrahepatic cholangiocarcinoma cells and tissues (Figure 7, P<0.05).
Figure 6.
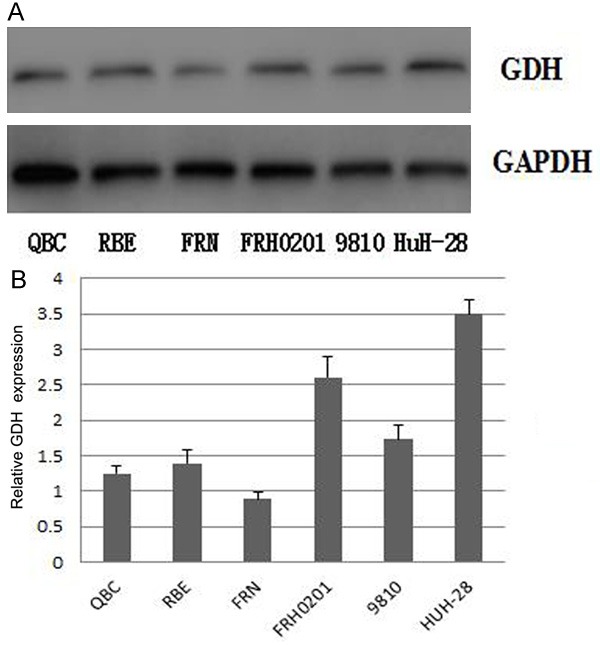
GDH expression before and after GDH silencing in QBC, RBE, FRN, FRH0201, 9810, and HuH-28 extrahepatic cholangiocarcinoma cells. A: western blotting results of GDH expression; B: Quantitative GDH expression levels in different cells.
Figure 7.
GDH expression in QBC cells and RBE cells at shGDH concentrations of 0 µg/ml, 5 µg/ml, 10 µg/ml, and 15 µg/ml. A: QBC cells; B: RBE cells.
GDH promotes the growth and metastasis of extrahepatic cholangiocarcinoma cells, potentially through TGF-β signal transmission mediated by the Smad signaling pathway
We established the GDH-overexpressing extrahepatic cholangiocarcinoma cell line QBC and determined whether GDH overexpression enhanced transmission of the Smad signaling pathway, resulting in decreased protein expression of Smad2, Smad3 and Smad4 and significantly increased protein expression of Smad7. After the QBC cells were treated with an anti-tumor agent, Smad2, Smad3 and Smad4 were significantly reduced, whereas Smad7 was significantly increased (Figure 8). These results demonstrate that GDH promotes extrahepatic cholangiocarcinoma cell growth and metastasis via TGF-β signal transmission mediated by the Smad signaling pathway.
Figure 8.
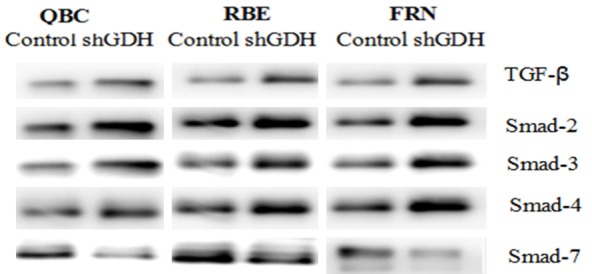
Smad protein expression levels in extrahepatic cholangiocarcinoma cells after retroviral GDH silencing or EGCG treatment. TGF-β signal transduction and protein expression levels of Smad2, Smad3, and Smad4 were significantly increased, and Smad7 protein expression was significantly reduced.
Discussion
The onset of extrahepatic cholangiocarcinoma is often insidious, with early diagnosis of extrahepatic cholangiocarcinoma particularly challenging. Early clinical manifestations of extrahepatic cholangiocarcinoma are often vague and not specific. Patients present for treatment jaundiced with elevated total serum bilirubin and tumor poses enormous difficulty for treatment and yields poor long-term patient outcomes [15,16]. Long-term survival of extrahepatic cholangiocarcinoma patients is closely related to the complete removal of the tumor [17]. With continuous improvements in imaging and surgical technology, the resection rate is also increasing. However, radical resection rates remained relatively low, with an average rate of 34.8% (16%-64%) [18].
TGF-β can significantly inhibit the growth of epithelial cells and lymphocytes, whereas the majority of human cancer cells are characterized by dysregulation of proliferation and an unlimited increase in the number of cells [19]. TGF-β was regarded as a tumor suppressor gene, but subsequent research has implicated TGF-β also modulates cancer microenvironments during the late development of certain tumors, and an elevated serum level of TGF-β significantly correlated with poor prognosis [20,21]. These findings suggest that TGF-β plays a complex role in tumor development. Prior to malignant cellular transformation, TGF-β plays an important role in tumor suppression by inducing growth arrest and apoptosis of epithelial cells, maintaining genomic stability, inhibiting telomerase activity and inducing senescence. However, during tumor progression, the cancer-promoting effects of TGF-β become dominant. TGF-β induces epithelial-mesenchymal transition (EMT) in tumor cells through direct action to increase cellular invasive potential [22]; TGF-β also promotes tumor growth by affecting the tumor stroma, suppressing immune surveillance and promoting the formation of new blood capillaries [23]. Smads play a key role in the TGF-β signaling pathway. Expression changes or dysfunction of Smad can significantly affect TGF-β pathway.
There is a lack of knowledge regarding the pathogenesis of extrahepatic cholangiocarcinoma, disease progression and non-surgical treatment alternatives. Consequently, there has been no significant improvement in extrahepatic cholangiocarcinoma survival in recent decades. In this study, we employed a sample set comprising a large cohort of cases to establish and improve clinical data systems, collect paraffin-embedded biopsy specimens, construct a high-throughput tissue microarray and launch cell biology and genomics-related research in extrahepatic cholangiocarcinoma. We believe that this study will deepen the awareness of the disease and improve survival of extrahepatic cholangiocarcinoma patients.
Initially, we established a large sample of clinical data on extrahepatic cholangiocarcinoma and 155 paraffin-embedded biopsy specimens from pathological specimens of extrahepatic cholangiocarcinoma were obtained by surgical excision. Immunohistochemical staining revealed increased GDH expression in extrahepatic cholangiocarcinoma to various degrees compared with normal bile duct tissue. The level of GDH expression in extrahepatic cholangiocarcinoma was closely related with CD34 positivity, cell differentiation, capsular and vascular invasion, lymph node metastasis, neural invasion and patient age. Single-factor and multi-factor survival analysis indicated that the prognosis of extrahepatic cholangiocarcinoma patients was closely associated with GDH expression (HR 3.80; 95% CI: 1.51-9.90; P=0.005), CD34 positivity (HR 2.02; 95% CI: 1.36-3.01; P=0.001), nerve infiltration (HR 2.55; 95% CI: 1.58-4.11; P=0.000) and cell differentiation (HR 0.001 95% CI: 0.000-0.006 P=0.000, HR 0.12 95% CI: 0.003-0.059; P=0.000). Therefore, the expression of GDH may be an important prognostic marker and therapeutic target for extrahepatic cholangiocarcinoma.
MTT assays of GDH-silenced and control cells revealed that silencing GDH significantly inhibited cell proliferation and the growth of adherent cells compared with the control group. Scratch testing indicated that silencing GDH significantly decreased the migratory ability of extrahepatic cholangiocarcinoma cells compared with the control group. Transwell assays demonstrated that silencing GDH significantly decreased the invasive potential of extrahepatic cholangiocarcinoma cells, and flow cytometry demonstrated that the cellular apoptosis was significantly higher after silencing GDH. qRT-PCR analysis and Western blotting revealed that both mRNA and protein expression of GDH were significantly lower after GDH silencing (both P<0.05).
Smads play a key role in the TGF-β signaling pathway. Aberrant expression or dysfunction of Smad can significantly affect the TGF-β pathway. To verify this relationship in extrahepatic cholangiocarcinoma cells, we employed either GDH silencing via retroviral transfection or added the tumor cell transduction inhibitor EGCG. Both methods significantly increased Smad2, Smad3 and Smad4 protein expression and significantly reduced Smad7 protein expression, which promoted the signal transduction of TGF-β via the Smad signaling pathway. In addition, blocking the Smad signaling pathway significantly promoted the proliferation and invasive/migratory potential of extrahepatic cholangiocarcinoma cells. We also established a GDH-overexpressing extrahepatic cholangiocarcinoma cell line, QBC, and determined that GDH overexpression can enhance transmission of the Smad signaling pathway. The protein expression levels of Smad2, Smad3 and Smad4 were significantly reduced in QBC cells, whereas Smad7 was significantly increased. After QBC cells were treated with the anti-tumor agent EGCG, Smad2, Smad3 and Smad4 were significantly reduced, whereas Smad7 was significantly increased.
Conclusions
The present study investigated GDH expression levels and biological function, and evaluated potential correlations with prognosis and the therapeutic value of targeting the Smad pathways regulated by GDH in extrahepatic cholangiocarcinoma. These findings demonstrate that GDH promotes extrahepatic cholangiocarcinoma cell growth and metastasis via TGF-β signal transmission mediated by the Smad signaling pathway.
Acknowledgements
This study was supported by Major Projects on Collaborative Innovation of Industry, Guangzhou (No.201508020076). This work was also supported by Grant [2013] 163 from Key Laboratory of Malignant Tumor Molecular Mechanism and Translational Medicine of Guangzhou Bureau of Science and Information Technology; Grant from the Key Laboratory of Malignant Tumor Gene Regulation and Target Therapy of Guangdong Higher Education Institutes (KLB09001). We gratefully acknowledge the assistance of the Department of Hepatobiliary Surgery for their help in collecting medical records. In addition, we express our thanks to all the participants in the study.
Disclosure of conflict of interest
None.
References
- 1.Ye XH, Huai JP, Ding J, Chen YP, Sun XC. Smoking, alcohol consumption, and the risk of extrahepatic cholangiocarcinoma: a meta-analysis. World J Gastroenterol. 2013;19:8780–8788. doi: 10.3748/wjg.v19.i46.8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173–184. doi: 10.1002/hep.24351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang H, Zhou J, Wei X, Wang F, Zhao H, Li E. Survival outcomes and progonostic factors of extrahepatic cholangiocarcinoma patients following surgical resection: adjuvant therapy is a favorable prognostic factor. Mol Clin Oncol. 2014;2:1069–1075. doi: 10.3892/mco.2014.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramirez-Merino N, Aix SP, Cortes-Funes H. Chemotherapy for cholangiocarcinoma: an update. World J Gastrointest Oncol. 2013;5:171–176. doi: 10.4251/wjgo.v5.i7.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carriaga MT, Henson DE. Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer. 1995;75:171–190. doi: 10.1002/1097-0142(19950101)75:1+<171::aid-cncr2820751306>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–125. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 7.Krstic J, Trivanovic D, Mojsilovic S, Santibanez JF. Transforming growth factor-Beta and oxidative stress interplay: implications in tumorigenesis and cancer progression. Oxid Med Cell Longev. 2015;2015:654594. doi: 10.1155/2015/654594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han J, Alvarez-Breckenridge CA, Wang QE, Yu J. TGF-beta signaling and its targeting for glioma treatment. Am J Cancer Res. 2015;5:945–955. [PMC free article] [PubMed] [Google Scholar]
- 9.Massague J, Chen YG. Controlling TGF-beta signaling. Genes Dev. 2000;14:627–644. [PubMed] [Google Scholar]
- 10.Itoh S, Itoh F, Goumans MJ, Ten DP. Signaling of transforming growth factor-beta family members through Smad proteins. Eur J Biochem. 2000;267:6954–6967. doi: 10.1046/j.1432-1327.2000.01828.x. [DOI] [PubMed] [Google Scholar]
- 11.Yang H, Xiong Y, Guan K. Metabolic alteration in tumorigenesis. Sci China Life Sci. 2013;56:1067–1075. doi: 10.1007/s11427-013-4549-2. [DOI] [PubMed] [Google Scholar]
- 12.Yang L, Moss T, Mangala LS, Marini J, Zhao H, Wahlig S, Armaiz-Pena G, Jiang D, Achreja A, Win J, Roopaimoole R, Rodriguez-Aguayo C, Mercado-Uribe I, Lopez-Berestein G, Liu J, Tsukamoto T, Sood AK, Ram PT, Nagrath D. Metabolic shifts toward glutamine regulate tumor growth, invasion and bioenergetics in ovarian cancer. Mol Syst Biol. 2014;10:728. doi: 10.1002/msb.20134892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu W, Le A, Hancock C, Lane AN, Dang CV, Fan TW, Phang JM. Reprogramming of proline and glutamine metabolism contributes to the proliferative and metabolic responses regulated by oncogenic transcription factor c-MYC. Proc Natl Acad Sci U S A. 2012;109:8983–8988. doi: 10.1073/pnas.1203244109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon HA, Noh MH, Kim BG, Han JS, Jang JS, Choi SR, Jeong JS, Chun JH. Clinicopathological significance of altered Notch signaling in extrahepatic cholangiocarcinoma and gallbladder carcinoma. World J Gastroenterol. 2011;17:4023–4030. doi: 10.3748/wjg.v17.i35.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang JW, Chu YM, Lan ZM, Tang XL, Chen YT, Wang CF, Che X. Correlation between metastatic lymph node ratio and prognosis in patients with extrahepatic cholangiocarcinoma. World J Gastroenterol. 2015;21:4255–4260. doi: 10.3748/wjg.v21.i14.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuller CD, Wang SJ, Cho M, Czito BG, Cornell J, Welzel TM, McGlynn KA, Luh JY, Thomas CR Jr. Multimodality therapy for locoregional extrahepatic cholangiocarcinoma: a population-based analysis. Cancer. 2009;115:5175–5183. doi: 10.1002/cncr.24572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon HJ, Kim SG, Chun JM, Lee WK, Hwang YJ. Prognostic factors in patients with middle and distal bile duct cancers. World J Gastroenterol. 2014;20:6658–6665. doi: 10.3748/wjg.v20.i21.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van der Gaag NA, Kloek JJ, de Bakker JK, Musters B, Geskus RB, Busch OR, Bosma A, Gouma DJ, van Gulik TM. Survival analysis and prognostic nomogram for patients undergoing resection of extrahepatic cholangiocarcinoma. Ann Oncol. 2012;23:2642–2649. doi: 10.1093/annonc/mds077. [DOI] [PubMed] [Google Scholar]
- 19.Jiang HL, Sun HF, Gao SP, Li LD, Hu X, Wu J, Jin W. Loss of RAB1B promotes triple-negative breast cancer metastasis by activating TGF-beta/SMAD signaling. Oncotarget. 2015;6:16352–16365. doi: 10.18632/oncotarget.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagpal N, Ahmad HM, Chameettachal S, Sundar D, Ghosh S, Kulshreshtha R. HIF-inducible miR-191 promotes migration in breast cancer through complex regulation of TGFbeta-signaling in hypoxic microenvironment. Sci Rep. 2015;5:9650. doi: 10.1038/srep09650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derynck R, Akhurst RJ, Balmain A. TGF-beta signaling in tumor suppression and cancer progression. Nat Genet. 2001;29:117–129. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 22.Papageorgis P. TGFbeta signaling in tumor initiation, epithelial-to-mesenchymal transition, and metastasis. J Oncol. 2015;2015:587193. doi: 10.1155/2015/587193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao L, Lin L, Pan C, Shi M, Liao Y, Bin J, Liao W. Flotillin-2 promotes nasopharyngeal carcinoma metastasis and is necessary for the epithelial-mesenchymal transition induced by transforming growth factor-beta. Oncotarget. 2015;6:9781–9793. doi: 10.18632/oncotarget.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]