Abstract
A bi-directional relationship between epilepsy and migraine has been widely reported in epidemiological and clinical studies, but the mechanisms of interaction between these disorders have not been fully examined using animal models. The aim of the present study was to develop a new comorbidity model of migraine and epilepsy. Nociception was induced by applying an inflammatory soup to the dura mater; this procedure resulted in nociception similar to that expressed in inflammatory disorders such as migraine. We showed that nociceptive behaviors (head rubbing, including with the forepaws and hindpaws) were significantly enhanced in comorbidity rats compared to sham-operation (Sham) rats, and these nociceptive behaviors were correlated with epilepsy-like behaviors. The plasma levels of calcitonin gene-related peptide (CGRP) significantly increased in the migraine group compared with the sham group, but CGRP did not further increase in comorbidity rats compared with migraine rats. C-Fos immunoreactive area values were higher in the comorbidity group than in the migraine group in the trigeminal nucleus caudalis (Sp5C) area (P<0.05). There was no significant difference in HSP70 expression between the comorbidity group and the epilepsy group. The expression of brain-derived neurotrophic factor (BDNF) in the CA3 area of the hippocampus in the comorbidity group was significantly higher than that in the epilepsy group according to the immunohistochemical test (P<0.05). In conclusion, our findings indicate that pilocarpine-induced epilepsy exacerbates inflammatory nociception, which can aggravate seizure severity. Moreover, we established a new comorbidity model for migraine and epilepsy.
Keywords: Comorbidity, migraine, epilepsy, model
Introduction
Epilepsy and migraine are chronic paroxysmal neurologic disorders [1] showing high comorbidity. The prevalence of epilepsy in Europe is estimated as 0.3-0.8% [2], and the prevalence of migraine is 11-15% [3-5]. Among patients with migraine, 6% have epilepsy, and up to 26% of patients with epilepsy have migraine [6-9]. A large epidemiological study revealed that adult persons with epilepsy were 2.4 times more likely to develop migraine than their relatives without epilepsy [10].
The cholinergic (muscarinic) agonist pilocarpine or lithium-pilocarpine model is widely used to characterize the mechanisms of status epilepticus (SE) resistance and the long-term consequences of SE, including neurodegenerative brain alterations, psychopathology, cognitive impairment and epilepsy, and determine how to prevent such sequelae with potentially antiepileptogenic compounds [11-14].
Inflammatory soup (IS), comprising a mixture of prostaglandin E2, serotonin, bradykinin, and histamine, has been frequently used in migraine studies to examine the activation of nociceptors in response to immune-derived products commonly released at sites of inflammation, including the meninges. The administration of inflammatory soup over the meninges induces the expansion of mechanical receptive fields in the skin, the sensitization of nociceptors to mechanical stimulation, and the sensitization of trigeminal nucleus caudalis neurons in anesthetized animals [15]. The inflammatory soup model has been successfully used to elucidate the mechanistic relationship between meningeal nociceptor activation by immune mediators and consequent changes in trigeminal sensory processing. Accordingly, the migraine model in the present study was elicited by IS.
To our knowledge, there is no established animal model on the association between epilepsy and migraine. In the present study, we investigated the effects of epilepsy on trigeminovascular nociception behaviors in conscious rats and determined the levels of calcitonin gene-related peptide (CGRP), brain-derived neurotrophic factor (BDNF), c-Fos and HSP70 to explore the molecular biological mechanisms of the comorbidity between epilepsy and migraine.
Materials and methods
Animals
Male Sprague-Dawley (SD) rats (Animal Center of Wuhan University, PR China) weighing 220-250 g were used. All rats were housed individually at a constant temperature (22±2°C) under standard lighting conditions (12/12-h dark/light cycle with the lights turned on at 07:00 am) and habituated in the experimental room for 1 hour per day for at least 1 week prior to the experiment. The experimental procedures were approved by the Committee on Animal Use for Research and Education of the Laboratory Animals Center, Renmin Hospital of Wuhan University (Wuhan, PR China), and were consistent with the ethical guidelines recommended by the International Association for the Study of Pain in Conscious Animals [16]. Efforts were made to minimize animal suffering.
Cannula implantation
After a midline incision, a guide cannula used for the dural delivery of IS or PBS was affixed on the dura mater adjacent to the superior sagittal sinus in all rats. IS (1 mM bradykinin, serotonin, and histamine and 0.1 mM prostaglandin E2 or 2 mM bradykinin, serotonin, and histamine and 0.2 mM prostaglandin E2; pH 5.5) was made fresh immediately prior to use from stock solutions. The animals with cannulas received IS (volume of injection: 30 µl) or PBS (volume of injection: 30 µl) through the cannula.
SE induction
The induction of status epilepticus using pilocarpine was performed as previously described [17]. Atropine (dissolved in 0.9% saline, Sigma Chemical) was subcutaneously administered at 1 mg/kg for 30 min prior to pilocarpine administration to reduce peripheral cholinergic effects. The animals received a single intraperitoneal (i.p.) dose of pilocarpine (250 mg/kg dissolved in 0.9% saline, Sigma Chemical). When the dose of pilocarpine was increased to 30 mg/kg, the rats became too sick to continue the following test and the mortality rate was high after 24 hours. When the dose of pilocarpine was decreased to 20 mg/kg, the number of the rats developing SE was too small to perform the subsequent experiment. Hence, 25 mg/kg was determined as the most efficient dosage.
The Racine scale, as previously described, was used with the following stages: (0) no abnormality, (1) mouth and facial movements, (2) head nodding, (3) forelimb clonus, (4) rearing, and (5) rearing and falling. A full motor seizure, with temporary loss of postural control, referred to as a stage 5 motor seizure [18], was used to index seizure intensity. Only rats displaying SE (stages 4-5) were selected. After the rats reached stage 5, some rats jumped up and down. Those rats were much more seriously affected than the rats reaching stage 5. We referred to this state as the jumping-up-and-down state.
Nociceptive behavior
A nociception scale was used, with face rubbing considered as nociceptive behavior. The nociception scale was measured using a von-Frey filament (vFF). The vFF can be used in paw withdrawal pain threshold assessment, one of the most popular tests for pain evaluation using animal models. For this test, a set of filaments, each exerting a different force, was applied to the rat skins immediately above the eyes, from the weakest to the strongest, until the rat head was withdrawn. This method starts with a mid-range filament and increases or decreases linearly, depending on the response of the animal. A defensive response results in the subsequent use of a lower filament, while a lack of a response dictates the use of a higher-strength filament for the next test.
As previously described, migraineurs commonly describe facial allodynia during a pain attack, including pain during hair brushing or face shaving [19]. Similarly, rats rub the face with forepaws and hindpaws when they receive IS.
Enzyme-linked immunosorbent assay (ELISA)
The levels of CGRP and BDNF in jugular blood [17,19] were determined using the ELISA method with aCGRP detection kit (Catal. No. CEA876Ra USCN, China) and aBDNF detection kit (Catal. No. SEA011Hu USCN, China) according to the manufacturer’s instructions. For jugular blood collection, 1 ml of blood sample in each group was drawn from the jugular vein and stored in Eppendorf tubes containing EDTA (1 mg/ml blood) and the protease inhibitor Aprotinin (0.55 TIU/ml blood).
Immunohistochemical detection
For immunohistochemical assays, sections of Sp5C tissues for c-Fos and the CA3 of hippocampus tissues for BDNF and HSP70 from different groups were incubated at 60°C overnight prior to dewaxing with dimethylbenzene. The slides were hydrated with alcohol, followed by washing with H2O2 for 5 min, fixing using methanol solution with 3% H2O2, and blocking with 1% goat serum for 15 min at room temperature. Subsequently, the slides were incubated with primary anti-c-Fos antibody (1:200), anti-BDNF antibody (1:200), and anti-HSP70 antibody (1:200) at 37°C for 30 min prior to incubation at 4°C overnight. After four cycles of washing with 0.01 mol/l PBS at 5 min for each cycle, secondary antibody (1:200) was added to the slides and placed at 37°C for 30 min prior to another four cycles of PBS wash. Slides were incubated with HRP at 37°C for 30 min prior to three cycles of 5-min PBS washing. Subsequently, DAB was added to the slides, followed by incubation for 3-10 min until the reaction was stopped using ddH2O. The slides were re-stained using hematoxylin and subsequently dehydrated. The percentage of positively stained cells and the staining intensity of the different groups were determined through observation under a microscope at 200× magnification by experimenters blind to the experiments.
Experiment procedure
The animals for the nociceptive behavior tests were treated according to Table 1. Ten minutes after the comorbidity group reached stage 4 on day 2, the comorbidity rats received chloral hydrate (30 mg/kg) to decrease the mortality rate. The nociceptive behavior tests were video recorded for 2 h on day 5 post-IS/PBS injection.
Table 1.
The animals for nociceptive behavior tests
| Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
|---|---|---|---|---|---|---|
| Migraine | Surgery | - | IS | IS | IS | IS |
| Comorbidity | Surgery | Pilocarpine + chloral hydrate | IS | IS | IS | IS |
| Sham | Surgery | - | PBS | PBS | PBS | PBS |
The animals for the seizure attack behavior tests were treated according to Table 2, and the tests were video recorded after the injection of pilocarpine. Additionally, pilocarpine and IS/PBS were simultaneously injected on day 5.
Table 2.
The animals for seizure attack behavior tests
| Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
|---|---|---|---|---|---|---|
| Pilo-surgery | Surgery | PBS | PBS | PBS | PBS | PBS + pilocarpine |
| Comorbidity | Surgery | IS | IS | IS | IS | IS + pilocarpine |
| Sham | Surgery | - | - | - | - | Pilocarpine |
The animals for ELISA tests were treated according to Table 3. The plasma samples were obtained at 2 h after the rats were administered a simultaneous injection the pilocarpine/IS/PBS on day 5. The animals for immunohistochemical tests were also tested according to Table 3. The immunohistochemical assays were performed at 2 h after the rats received pilocarpine/IS/PBS treatment on day 5.
Table 3.
The animals for ELISA tests and immunohistochemicaltests
| Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
|---|---|---|---|---|---|---|
| Migraine | Surgery | - | - | - | - | IS |
| Pilo-surgery | Surgery | - | - | - | - | Pilocarpine + PBS |
| Comorbidity | Surgery | - | - | - | - | Pilocarpine + IS |
| Sham | Surgery | - | - | - | - | PBS |
Statistical analysis
Prism 5 were used for statistical analyses and to generate graphs. One-way ANOVA for multiple comparisons was used to compare differences among groups. Post-hoc comparisons between experimental groups were performed using the least significant difference test. A chi-square test was used to calculate significant differences in the incidence of jumping-up-and-down state. The data are presented as the means ± SEM. A value of P<0.05 indicated statistical significance.
Results
The effects of pilocarpine-induced seizure attack on nociceptive behaviors
The animals were prepared according to Table 1. The rats in the migraine group without pilocarpine (219.8±20.69) showed a significantly higher number of rubbings than the sham group (145.9±16.93) (P=0.0204) (Figure 1A). These results showed that the migraine model used in the present study was successfully established.
Figure 1.
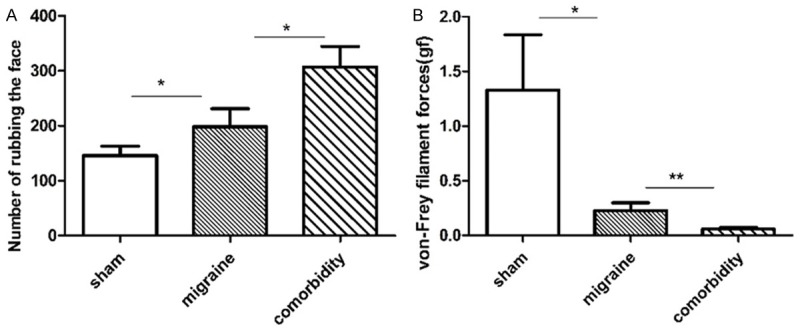
The effects of pilocarpine-induced seizure attacks on nociceptive behavior. A: Number of face rubbings. The number of face rubbings significantly increased in the migraine group compared with the sham group. The number of face rubbings markedly increased in the comorbidity group compared with those in the migraine group (P=0.0176; N=15 for sham, N=12 for migraine, N=12 for comorbidity). B: Von Frey filament forces. The von Frey filament forces were significantly lower in the comorbidity group than in the migraine group. N=12 for sham, migraine and comorbidity. *P<0.05, **P<0.01. Note: One-way ANOVA followed by Student’s t test was used for comparisons between groups.
As shown in Figure 1A and 1B, the number of face rubbings markedly increased in rats in the comorbidity group (307.2±37.45) compared with those in the migraine group (198.1±33.24) (P=0.0176). The von-Frey filament forces markedly increased in rats in the sham group compared with those in the migraine group (P=0.0172) (Figure 1B).
The effects of IS-induced migraine on seizure attacks
Animals were prepared according to Table 2. The rats in the comorbidity group displayed significantly decreased latent periods (1521±93.62) than those in the pilo-surgery group pretreated with PBS (2055±143.0) during the experiment (P=0.0023) (Figure 2A). An increased number of rats in the comorbidity group reached the jumping-up-and-down state than in the sham group pre-treated with PBS (100% vs. 50%) (Figure 2B).
Figure 2.
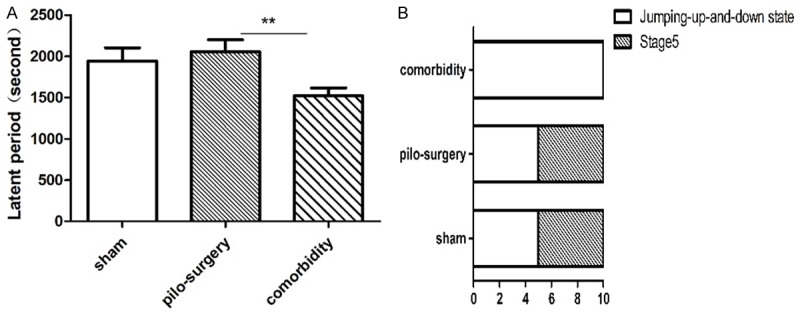
The effects of IS-induced migraine on seizure attacks (A). In contrast to the epilepsy group pre-treated with PBS, the rats in the comorbidity group displayed a decreased latent period. N=12 for sham, N=18 for pilo-surgery, N=24 for comorbidity. (B) Racine scale. More rats in the comorbidity group reached the jumping-up-and-down state than in the pilocarpine-induced seizure attack group pretreated with PBS. N=10 for sham, pilo-surgery, and comorbidity. *P<0.05, **P<0.01. Note: One-way ANOVA followed by Student’s t test was used for comparisons between groups for (A). Chi-square test was used in (B).
The effects of pilocarpine-induced seizure attack on CGRP levels and the effects of IS-induced migraine on BDNF levels
Animals were prepared according to Table 3. Figure 3A shows the blood concentration of CGRP in the external jugular vein. The CGRP level in the migraine group (324.9±2.53) was significantly higher than that in the sham group (81.74±81.7) (P=0.0079), whereas the CGRP level in the comorbidity group was lower than that in the migraine group. These results might demonstrate that the comorbidity group became seriously sick and could not accurately feel pain. The behavior of the comorbidity group was significantly different from that of the migraine group. The results for plasma levels and behavior were not similar because the recorded time points were different.
Figure 3.
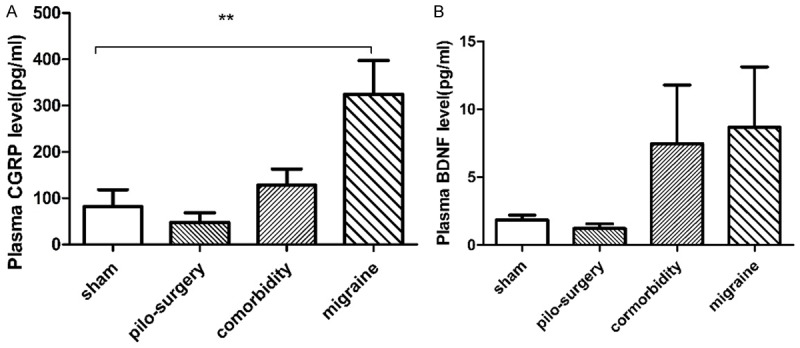
(A) The effects of pilocarpine-induced seizure attack on CGRP levels. The CGRP level in the migraine group was significantly higher than that in the sham group, whereas the CGRP level in comorbidity group was lower than that in the migraine group. N=10. (B) The effects of IS-induced migraine on BDNF levels. There was no significant difference among the sham, migraine, pilocarpine-induced seizure attack and comorbidity groups. N=10. *P<0.05, **P<0.01. Note: One-way ANOVA followed by Student’s t test was used for comparisons between groups.
There was no significant difference among the sham, migraine, and comorbidity groups in plasma BDNF levels (Figure 3B).
The effects of pilocarpine-induced seizure attack on c-Fos levels and the effects of IS-induced migraine on HSP70 and BDNF levels
The animals were prepared according to Table 3. As shown in Figure 4A, c-Fos-positive neurons were determined as brown granules in the cell nucleus. One-way ANOVA followed by Student’s t test was used for comparisons between groups. The expression of c-Fos in the comorbidity group (110.8±1101) was higher than that in the migraine group in the Sp5C (78.12±3.159) in the trigeminal sensory nuclear complex, and the expression of c-Fos in migraine group (78.12±3.159) was significantly higher than that in the sham group (61.23±5.606) (P=0.0207) (Figure 4B).
Figure 4.
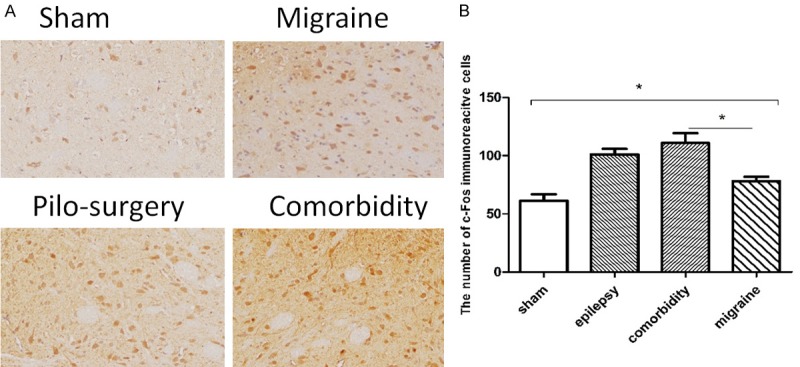
The effects of pilocarpine-induced seizure attack on c-Fos levels. A: The expression of c-Fos in four groups in the Sp5C. c-Fos-positive neurons were determined as brown granules in the cell nucleus. B: The c-Fos expression in the comorbidity group was higher than that in the migraine group in the Sp5C in the trigeminal sensory nuclear complex, and the expression of c-Fos in the migraine group was significantly higher than that in the sham group. N=6. *P<0.05. Note: One-way ANOVA followed by Student’s t test was used for comparisons between groups.
As shown in Figure 5A, HSP70-positive neurons were determined by the presence of brown granules in the cell nucleus. The expression of HSP70 in the comorbidity group was higher than that in the sham group. However, the expression of HSP70 in the comorbidity group was not significantly higher than that in the pilocarpine-induced seizure attack group (Figure 5B). As shown in Figure 6A, BDNF-positive neurons were shown as brown granules in the cytoplasm. The BDNF expression in the comorbidity group (1588±384.6, 4596±243.7) was significantly higher than that in the pilocarpine-induced seizure attack group (1588±384.6) (Figure 6B).
Figure 5.
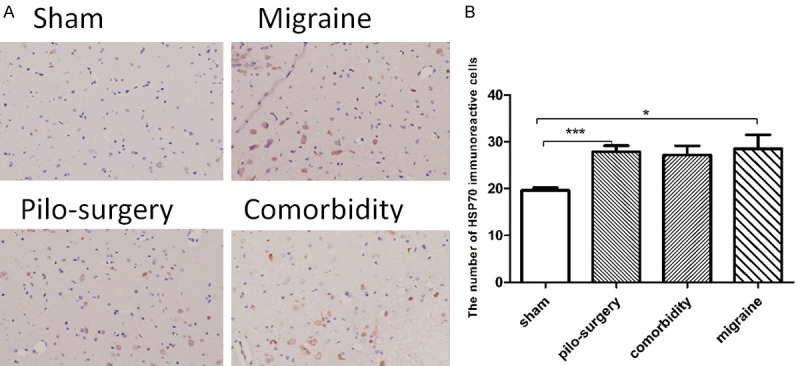
The effects of IS-induced migraine on HSP70 levels. A: The expression of HSP70 in the four groups in the CA3 area of the hippocampus. HSP70-positive neurons were determined as brown granules in the cell nucleus. B: The expression of HSP70 in the pilocarpine-induced seizure attack group was higher than that in the sham group. N=6. *P<0.05, **P<0.01, ***P<0.001. Note: One-way ANOVA followed by Student’s t test was used for comparisons between groups.
Figure 6.
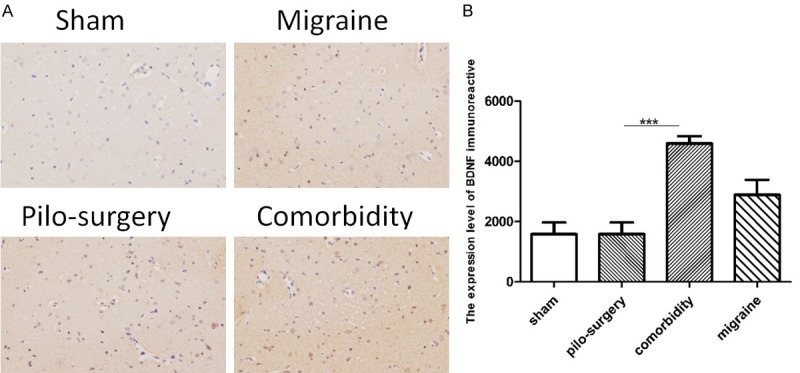
The effects of IS-induced migraine on BDNF levels. A: The expression of BDNF in four groups in the hippocampus. BDNF-positive neurons were determined as brown granules in the cytoplasm. B: The expression of BDNF in the comorbidity group was significantly higher than that in the pilocarpine-induced seizure attack group pretreated with PBS. N=6. *P<0.05, **P<0.01, ***P<0.001. Note: One-way ANOVA followed by Student’s t test was used for comparisons between groups.
Discussion
Epilepsy is a paroxysmal disease comorbid with migraine, depression, anxiety, cognitive impairment and attention deficits. The comorbidity of headache and epilepsy is well known: the frequency of epilepsy among people with migraine (range 1-17%) is higher than that in the general population (0.5-1%), just as the prevalence of migraine among patients with epilepsy is higher (range 8-15%) than that reported in healthy individuals [20]. A survey study of 597 patients from 32 epilepsy clinics showed that the age at the onset of epilepsy was lower in patients with seizure-related headaches than in those without, and the risk of seizure-related headaches was significantly higher in patients with longer epilepsy duration. The comorbidity between migraine and epilepsy decreases quality of life, complicates treatment, and increases mortality. There are comorbidity models of epilepsy and depression and of epilepsy and anxiety, but there is no comorbidity model of epilepsy and migraine. However, such a model could help us to better understand the mechanisms of comorbidity. The aim of the present study was to establish a new comorbidity model. The comorbidity model exhibited much more rubbing than the migraine rats and greater latency to seizures than the epilepsy rats (Figures 2 and 3). These results might help us understand why migraineurs complain of a higher frequency of headaches during bouts of epilepsy. Additionally, the data may help us better understand why epileptics undergo more serious attacks when they have a migraine. Subsequently, the comorbidity worsened both migraine and epilepsy.
CGRP, a neuropeptide released from activated trigeminal sensory nerves, dilates intracranial blood vessels and transmits vascular nociception. Interestingly, plasma levels of CGRP, but not other neuropeptides, were elevated in the external jugular vein during the headache phase of migraine, and these levels were normalized to triptans in parallel with the amelioration of headache [21]. Therefore, a clear association exists between CGRP and migraine [22], and CGRP is a biological marker of trigeminovascular activation [21,22]. In addition, in the present study, the GGRP in the external jugular vein significantly increased in the comorbidity group compared with the levels in the single migraine group. The results showed that the migraine rats experienced more serious pain than the sham rats. However, the CGRP in the comorbidity rats was lower than that in the migraine rats. This result might reflect the fact that the plasma of the comorbidity rats was sampled only 2 h after these rats received IS and pilocarpine. The behavior of the comorbidity group was significantly different from that of the migraine group. The results of plasma and behavior were not similar because we recorded different time points.
The c-Fos gene is an early-response gene. c-Fos is a sensitive marker of neuronal activation following noxious stimulation, and the expression of c-Fos has been widely used to identify areas of neuronal activation and to study the neural correlates of nociception [23]. c-Fos expression can be induced within the trigeminal nucleus via the application of different stimuli (mechanical, electrical or chemical) in either intra- or extra cranial structures innervated by the trigeminal nerve. The Sp5C is a component of the ascending sensory pathway that transmits nociceptive signals from the trigeminal nerve territory to the thalamus. Nociceptive information from the orofacial region is input to the Sp5C and subsequently transmits to the ventral posteromedial (VPM) nucleus of the thalamus [24]. The results demonstrated that the expression of c-Fos in the Sp5C area increased in the migraine group compared with the sham group. The results of the present study are consistent with those of previous studies. In the present study, the expression of c-Fos in the Sp5C area significantly increased in the comorbidity group compared with the migraine group (Figure 4). The detection demonstrated that comorbidity involves more intense pain than simple migraine. Therefore, epilepsy worsened the pain.
Heat shock proteins (HSPs) are induced in response to many injuries, including stroke, neurodegenerative disease, epilepsy, and trauma. The overexpression of one HSP in particular, HSP70, serves a protective role in several different models of nervous system injury but has also been linked to a deleterious role in some diseases. HSP70 acts as a chaperone that protects neurons from protein aggregation and toxicity (Parkinson’s disease, Alzheimer’s disease, polyglutamine diseases, and amyotrophiclateral sclerosis), protects cells from apoptosis (Parkinson’s disease), is a stress marker (temporal lobe epilepsy), protects against inflammation (cerebral ischemic injury), has an adjuvant role in antigen presentation and is involved in the immune response in autoimmune diseases (multiple sclerosis) [25]. Inpatients with temporal lobe epilepsy, HSP70 can be considered as a potential stress biomarker because its serum level exhibits an inverse correlation with memory function and hippocampal volume. In the present study, there was no significant difference in the expression of HSP70 between the comorbidity group and the seizure attack group (Figure 5). Furthermore, the migraine rats (28.50±3.030) exhibited higher HSP70 than the sham animals (19.67±0.5578) (P=0.0168). No evidence has ever showed that HSP70 correlates with migraine. Nevertheless, in the present study, we detected that HSP70 increased in migraine rats. HSP70 is a protective protein in several nervous system injuries: thus, this protein might also play a protective role in migraine.
Previous evidence suggested that abnormal activity of brain-derived neurotrophic factor (BDNF) contributes to the pathogenesis of epilepsy [26]. According to several reports, the development of epilepsy can be attenuated or postponed through the intrahippocampal delivery of BDNF [27]. At the initial stage of epileptogenesis in temporal lobe epilepsy, seizures can increase the production of BDNF to foster neurogenesis, decrease neuronal cell death, and exert anti-inflammatory effects, leading to the protection of neurons against seizure-induced damage [26]. The ELISA results obtained in the present study did not reveal a significant difference among the groups (Figure 3). However, immunohistochemical analysis showed that the comorbidity rats had higher expression levels of BDNF in the CA3 area of the hippocampus than the pilocarpine-induced seizure attack rats. This finding might suggest that BDNF had not been released into the blood when we sampled the plasma. Therefore, the comorbidity rats might have more serious attacks than the simple seizure attack animals (Figure 6).
The limitation of the present study is that the sampling time point might be more optimal. The mechanisms underlying the comorbidity of migraine and epilepsy should be further studied. In the future, the common pathway underlying the comorbidity should be explored and a therapeutic target should be identified.
Conclusions
In summary, pilocarpine-induced epilepsy could exacerbate trigeminovascular nociception, making the rats more sensitive to pain. Moreover, IS-induced migraine could decrease the latency period of epilepsy rats and increase the risk of SE. Finally, we established a comorbidity model of migraine and epilepsy to help us better understand the mechanism.
Acknowledgements
National Natural Science Foundation of China (81471133, 30900459), The Ministry of Education Fund for new teachers (200804861046), and the Nature Science Fund of Hubei Province (2014CFB734).
Disclosure of conflict of interest
None.
References
- 1.Costa C, Prontera P, Caproni S, Cupini LM, Sarchielli P, Calabresi P. Migraine and epilepsy: what value today? J Headache Pain. 2015;16:A44. doi: 10.1186/1129-2377-16-S1-A44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forsgren L, Beghi E, Oun A, Sillanpaa M. The epidemiology of epilepsy in Europe-a systematic review. Eur J Neurol. 2005;12:245–253. doi: 10.1111/j.1468-1331.2004.00992.x. [DOI] [PubMed] [Google Scholar]
- 3.Rasmussen BK, Jensen R, Schroll M, Olesen J. Epidemiology of headache in a general population-a prevalence study. J Clin Epidemiol. 1991;44:1147–1157. doi: 10.1016/0895-4356(91)90147-2. [DOI] [PubMed] [Google Scholar]
- 4.Hagen K, Zwart JA, Vatten L, Stovner LJ, Bovim G. Prevalence of migraine and non-migrainous headache-head-HUNT, a large population-based study. Cephalalgia. 2000;20:900–906. doi: 10.1046/j.1468-2982.2000.00145.x. [DOI] [PubMed] [Google Scholar]
- 5.Bigal ME, Lipton RB. The epidemiology, burden, and comorbidities of migraine. Neurol Clin. 2009;27:321–334. doi: 10.1016/j.ncl.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Marks DA, Ehrenberg BL. Migraine-related seizures in adults with epilepsy, with EEG correlation. Neurology. 1993;43:2476–2483. doi: 10.1212/wnl.43.12.2476. [DOI] [PubMed] [Google Scholar]
- 7.Ottman R, Lipton RB. Comorbidity of migraine and epilepsy. Neurology. 1994;44:2105–2110. doi: 10.1212/wnl.44.11.2105. [DOI] [PubMed] [Google Scholar]
- 8.Tellez-Zenteno JF, Matijevic S, Wiebe S. Somatic comorbidity of epilepsy in the general population in Canada. Epilepsia. 2005;46:1955–1962. doi: 10.1111/j.1528-1167.2005.00344.x. [DOI] [PubMed] [Google Scholar]
- 9.Babu CS, Satishchandra P, Sinha S, Subbakrishna DK. Co-morbidities in people living with epilepsy: hospital based case-control study from a resource-poor setting. Epilepsy Res. 2009;86:146–152. doi: 10.1016/j.eplepsyres.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Lipton RB, Ottman R, Ehrenberg BL, Hauser WA. Comorbidity of migraine: the connection between migraine and epilepsy. Neurology. 1994;44:S28–S32. [PubMed] [Google Scholar]
- 11.Song PP, Xiang J, Jiang L, Chen HS, Liu BK, Hu Y. Dynamic changes in spectral and spatial signatures of high frequency oscillations in rat hippocampi during epileptogenesis in acute and chronic stages. Front Neurol. 2016;7:204. doi: 10.3389/fneur.2016.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li R, Ma L, Huang H, Ou S, Yuan J, Xu T, Yu X, Liu X, Yang J, Chen Y, Peng X. Altered expression of CXCL13 and CXCR5 in intractable temporal lobe epilepsy patients and pilocarpine-induced epileptic rats. Neurochem Res. 2017;42:526–540. doi: 10.1007/s11064-016-2102-y. [DOI] [PubMed] [Google Scholar]
- 13.Fu P, Wen Y, Xiong Y, Zhang Y, Zhang H, Xie Y, Shi Q. Abnormal expression of FBXL20 in refractory epilepsy patients and a pilocarpine-induced rat model. Neurochem Res. 2016;41:3020–3031. doi: 10.1007/s11064-016-2021-y. [DOI] [PubMed] [Google Scholar]
- 14.Liang JJ, Huang LF, Chen XM, Pan SQ, Lu ZN, Xiao ZM. Amiloride suppresses pilocarpine-induced seizures via ASICs other than NHE in rats. Int J Clin Exp Pathol. 2015;8:14507–14513. [PMC free article] [PubMed] [Google Scholar]
- 15.Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1998;79:964–982. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- 16.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 17.Liang J, Yu S, Dong Z, Wang X, Liu R, Chen X, Li Z. The effects of OB-induced depression on nociceptive behaviors induced by electrical stimulation of the dura mater surrounding the superior sagittal sinus. Brain Res. 2011;1424:9–19. doi: 10.1016/j.brainres.2011.09.048. [DOI] [PubMed] [Google Scholar]
- 18.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 19.Kang NI, Park JI, Kim YK, Yang JC. Decreased plasma BDNF levels of patients with somatization disorder. Psychiatry Investig. 2016;13:526–530. doi: 10.4306/pi.2016.13.5.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verrotti A, Coppola G, Spalice A, di Fonzo A, Bruschi R, Tozzi E, Iannetti P, Villa MP, Parisi P. Peri-ictal and inter-ictal headache in children and adolescents with idiopathic epilepsy: a multicenter cross-sectional study. Childs Nerv Syst. 2011;27:1419–1423. doi: 10.1007/s00381-011-1428-7. [DOI] [PubMed] [Google Scholar]
- 21.Goadsby PJ, Lipton RB, Ferrari MD. Migraine-current understanding and treatment. N Engl J Med. 2002;346:257–270. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- 22.Edvinsson L. Blockade of CGRP receptors in the intracranial vasculature: a new target in the treatment of headache. Cephalalgia. 2004;24:611–622. doi: 10.1111/j.1468-2982.2003.00719.x. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Zhang Q, Qi D, Zhang L, Yi L, Li Q, Zhang Z. Valproate ameliorates nitroglycerin-induced migraine in trigeminal nucleus caudalis in rats through inhibition of NF-кB. J Headache Pain. 2016;17:49. doi: 10.1186/s10194-016-0631-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayashi B, Maeda M, Tsuruoka M, Inoue T. Neural mechanisms that underlie angina-induced referred pain in the trigeminal nerve territory: a c-Fos study in rats. ISRN Pain. 2013;2013:671503. doi: 10.1155/2013/671503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turturici G, Sconzo G, Geraci F. Hsp70 and its molecular role in nervous system diseases. Biochem Res Int. 2011;2011:618127. doi: 10.1155/2011/618127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sha’ari HM, Haerian BS, Baum L, Tan HJ, Rafia MH, Kwan P, Cherny SS, Sham PC, Gui H, Raymond AA, Lim KS, Mohamed Z. Association of BDNF polymorphisms with the risk of epilepsy: a multicenter study. Mol Neurobiol. 2016;53:2869–2877. doi: 10.1007/s12035-015-9150-1. [DOI] [PubMed] [Google Scholar]
- 27.Eftekhari S, Mehrabi S, Karimzadeh F, Joghataei MT, Khaksarian M, Hadjighassem MR, Katebi M, Soleimani M. Brain derived neurotrophic factor modification of epileptiform burst discharges in a temporal lobe epilepsy model. Basic Clin Neurosci. 2016;7:115–120. doi: 10.15412/J.BCN.03070205. [DOI] [PMC free article] [PubMed] [Google Scholar]


