Abstract
This study was conducted to investigate the repair mechanism of hypoxia/reoxygenation injury (HRI) in renal tubular epithelial cells (HK-2) by exogenous mesenchymal stem cells (MSCs). The activation of the JAK/STAT pathway in HK-2 cells after HRI and treatment of MSCs, JAK inhibitor WP1066 and STAT inhibitor SOCS3 was investigated using Western blot analysis. HK-2 cells were transfected with siRNA STAT3 and analyzed for expression of STAT3, JAK2 and HMGB1 using fluorescence quantitative PCR and Western blot. Cell viability and apoptosis were analyzed using the MTT assay and flow cytometry. After HRI, the JAK/STAT pathway in HK-2 cells was activated, resulting in the upregulation of JAK1, JAK2, JAK3, p-JAK1, p-JAK2, p-JAK3, STAT1, STAT2, STAT3, p-STAT1, p-STAT2 and p-STAT3. After treatment with MSC conditioned medium (MSCs CM), WP1066, or SOCS, the expression of these proteins was significantly down-regulated. When the cells were transfected with siRNA STAT3, the expression of STAT3 at protein and mRNA levels and JAK2 and HMGB1 at mRNA level was down-regulated; the cell viability was reduced and apoptosis increased. It is concluded that exogenous MSCs reduce HRI of HK-2 cells by suppressing the JAK/STAT signaling pathway and down-regulating the expression of HMGB1.
Keywords: Mesenchymal stem cells, receptor tyrosine kinase, signal transducer and activator of transcription, high mobility group protein 1, hypoxia/reoxygenation injury
Introduction
Ischemia-reperfusion injury (IRI) is the tissue damage or function failure caused when blood supply returns to the tissue after a period of ischemia or lack of oxygen (hypoxia) [1-3]. Renal IRI is an inevitable inflammatory stage in renal surgery and transplantation. At present there is no effective method to reduce the renal IRI, and acute kidney injury in some of patients is unable to become chronic renal injury, leading eventually to end-stage renal disease. Studies of our group and others have demonstrated that mesenchymal stem cells (MSCs) can improve the repair of acute and chronic renal or tissue damages [1,2,4]. MSCs are multipotent, self-renewing cells that exist in the bone marrow, liver, umbilical cord and placenta. They are low immunogenic, easy to obtain, proliferate and store, and are gradually being used for treatment of acute and chronic renal diseases [1,2,4].
During renal IRI, a variety of inflammatory mediators are released, resulting in chemotaxis and accumulation of the neutrophils in the injury site and aggravation of the inflammatory reaction. High mobility group box 1 (HMGB1) is considered to be the most important inflammatory mediator in renal IRI. Studies showed that its expression is up-regulated in the early stage of IRI, reaches a peak as the perfusion continues and then declines. Its expression is further significantly increased when the tissue is reperfused [5,6]. Meanwhile, the pathological process in IRI is related to a number of inflammatory signal pathways, such as the JAK/STAT signal pathway [7-9]. HMGB1 is shown to mediate the inflammatory responses through the JAK-STAT signaling pathway [10,11]. Therefore, we hypothesized that MSCs may also regulate the expression of HMGB1 through the JAK/STAT signaling pathway to reduce the IRI in the kidney. In this study, we constructed a hypoxia and reoxygenation injury (HRI) model of renal tubular epithelial cells (HK-2), and treated the model with MSCs and the inhibitors of the JAK-STAT signaling pathway and analyzed the expression of proteins in the JAK-STAT signaling pathway. We also examined the expression of HMGB1 in STAT3-knocdown HK-2 cells. The findings will provide new insights into the molecular mechanism underlying MSCs-mediated reduction of renal IRI.
Materials and methods
Reagents
Polyclonal rabbit anti-JAK1, JAK2, JAK3, p-JAK1, p-JAK2, p-AK3, STAT1, STAT2, STAT3, p-STAT1, p-STAT2, p-STAT3 and β-actin antibodies were purchased from Abcam (USA); RNA extraction kit and fluorescence quantitative PCR kit, JAK inhibitor WP1066 and STAT inhibitor SOCS3 were purchased from CWbiotech (Beijing, China); Lipofectamine 2000 was from Invitrogen, USA; MTT and cell apoptosis detection kits were purchased from Keygen Biotech (Nanjing, China); siRNA plasmid and empty plasmid were from Riobio (Guangzhou, China); fetal bovine serum (FBS) and Dulbecco’s modified Eagle’s medium (DMEM) were purchased from GIBCO (USA).
Cell line
Rat HK-2 cell line was purchased from the Cell Bank, the Chinese Academy of Sciences (CM-H507, Shanghai, China).
Isolation, culture and purification of MSCs
The marrow cavities of bilateral femur and tibia of four-week old male Wistar rats were rinsed with DMEM medium under sterile conditions to obtain cell suspension and MSCs were obtained using gradient centrifugation as previously described [12]. MSCs were obtained with a protocol and procedures approved by the Institutional Animal Care and Use Committee. Cells were cultured in DMEM medium containing 10% FBS at 37°C and 5% CO2. The medium was first refreshed in 24 h and then every 3 days to gradually remove the non-adherent cells. When the cells reached 90% confluence, they were digested with 0.25% trypsin and passaged at least ten times before being used for experimentation.
Hypoxia/reoxygenation treatment
Hypoxia/reoxygenation treatment of HK-2 cells was performed as described [13,14]. Briefly, for hypoxia treatment, a mixture of 95% N2 and 5% CO2 was filled to the airtight containers that contain the cells at a flow rate of 10 L/min for 6 min, and the bottles were screwed, sealed and cultured for 4 h. For reoxgenation, the bottles were open for 10 min and then cultured for 10 h.
Cell culture and grouping
MSCs conditioned medium (MSCs-CM) was prepared. JAK inhibitor WP1066, STAT inhibitor SOCS3 were added to a final concentration of 10 μmol/L in various combinations: group A, saline + HK-2 IRI; group B, MSCs-CM + HK-2 IRI; group C, group B + WP1066; group D, group B + SOCS and group E, group B + WP1066 + SOCS.
Western blot analysis
Cells were treated and cultured for 48 h, harvested and lysed by adding the lysis buffer to extract total protein. The protein content was determined using the BCA kit and the proteins were separated on SDS gels by electrophoresis, transferred to membranes for Western blot analysis. The membranes were blocked for 1 h in block solution containing 5% defatted milk powder, and incubated in anti-body solutions overnight at 37°C; after rinsing, the membranes were immersed in a second antibody solution (horseradish peroxidase-labeled goat anti rabbit IgG) at room temperature for 1-2 h. The membranes were washed, developed by adding with ECL exposure solution, and captured for images using a gel imaging system (ChemiDocTM XRS, Biorad, USA). The gray values of the bands were determined using “One Quantity” software.
Design and construction of interfering vector
To generate STAT3 knockdown cells STAT3, siSTAT3 was designed according to the sequence of STAT3 to construct a RNA interfering vector pSilencer3.0-H1-STAT3-siRNA-GFP (Riobio, Guangzhou, China). The siRNA sequences were 5’-GCAGCAGCTGAACAACATGTTCAAGAGACATGTTGTTCAGCTGCTGCTTTTT-3’ (forward) and 5’-AATTAAAAAGCAGCAGCTGAACAACATGTCTCTTGAACATGTTGTTCAGCTGCTGCTGCGGCC-3’ (reverse).
Transfection
The RNAi vector and empty vector were transfected into HK-2 cells using the Lipofectamine 2000-mediated transfection methods [15]. The cells were cultured in DMEM medium for 72 h and harvested for assays.
Real-time PCR
Total RNA was extracted using the Trizol method. After the determination of purity, the RNA was reversely transcripted into cDNA and used for fluorescence quantitative PCR on CFX96 fluorescence quantitative PCR instrument (Biorad, USA) using the following primers: STAT3 upstream primer 5’-GTACTAGTGTCTCAGGACC C-3’ and downstream primer 5’-TTAAGCTTCTCATACGAGG-3’; JAK upstream primer 5’-ATCCA CCCAA CCATG TCTTC C-3’, downstream primer 5’-ATTCCATGCCGATAGGCTCTG-3’; HMGB1 upstream primer 5’-TCAAAGGAGAACATCCTGGCCTGT-3’ and downstream primer 5’-CTGCTTGTCATCTGCAGCAGTGTT-3-3’. β-actin was used as loading control and amplified with upstream primer 5’-AGCGAGCATCCCCCAAAGTT-3’ and downstream primer 5’-GGGCACGAAGGCTCATCATT-3’. The reverse transcription system contained 2 µL total RNA template (50 pg-5 µg), 4 µL dNTP mixture and 2 µL MgCl2. The reaction parameters were: pre-denaturing at 95°C for 10 min, followed by 40 cycles of denaturing at 95°C for 15 s, annealing at 60°C for 1 min and extension at 70°C for 30 s. The relative expression value was calculated using the 2-ΔΔCT method [16].
Cell viability assay
HK-2 cells were seeded in wells of 96 well plates after transfection or treatment, cultured for 48 h and added with 50 µL 1 × MTT buffer according to the manufacturer’s instructions. After incubation for 4 h, 150 µL DMSO was added to each well and optical density at 550 nm was determined.
Determination of apoptosis
HK-2 cells were harvested and collected by centrifugation. 1-5 × 105 cells were suspended in 500 µL binding buffer and stained with 5 µL Annexin V-FITC and 5 µL propidium iodide for 5-15 min at room temperature in the dark. The stained cells were subjected to flow cytometry analysis on FACSCalibar Flow Cytometer (Beckton Dickinson, USA).
Statistical analysis
All experiments were repeated at least three times and data were expressed as x̅±s. The difference was tested using the Student’s t-test and value with P < 0.05 was considered statistically significant. All the data were statistically analyzed using SPSS 17.
Results
HRI induces the activation of JAK/STAT signaling pathway in HK-2 cells
We first examined the expression of proteins in the JAK/STAT signaling pathway HK-2 cells after hypoxia/reoxygenation treatment. The Western blot analyses showed that the levels of Jak1, JAK2, JAK3, p-JAK1, p-JAK2, p-JAK3, STAT1, STAT2, STAT3 and p-STAT1, p-STAT2, p-STAT3 were significantly higher after the hypoxia/reoxygenation treatment as compared with untreated cells (P < 0.01, Figure 1).
Figure 1.

Levels of JAK/STAT signaling pathway proteins in HK-2 cells before (A) and after (B) hypoxia/reoxygenation treatment. Upper pane, representative Western blots; lower pane, relative protein levels. **P < 0.01 vs untreated cells.
Exogenous MSC-CM and pathway inhibitors down-regulate the expression of JAK/STAT signaling pathway proteins in HK-2 cells after hypoxia/reoxygenation treatment
We then analyzed the effect of exogenous MSCs-CM and pathway inhibitors on expression of these pathway proteins. In compared with the cells incubated with control, MSC-CM and pathway inhibitors WP 1066 and SOCS3 significantly reduced the levels of these proteins in HK-2 after hypoxia/reoxygenation treatment (P < 0.01, Figure 2).
Figure 2.
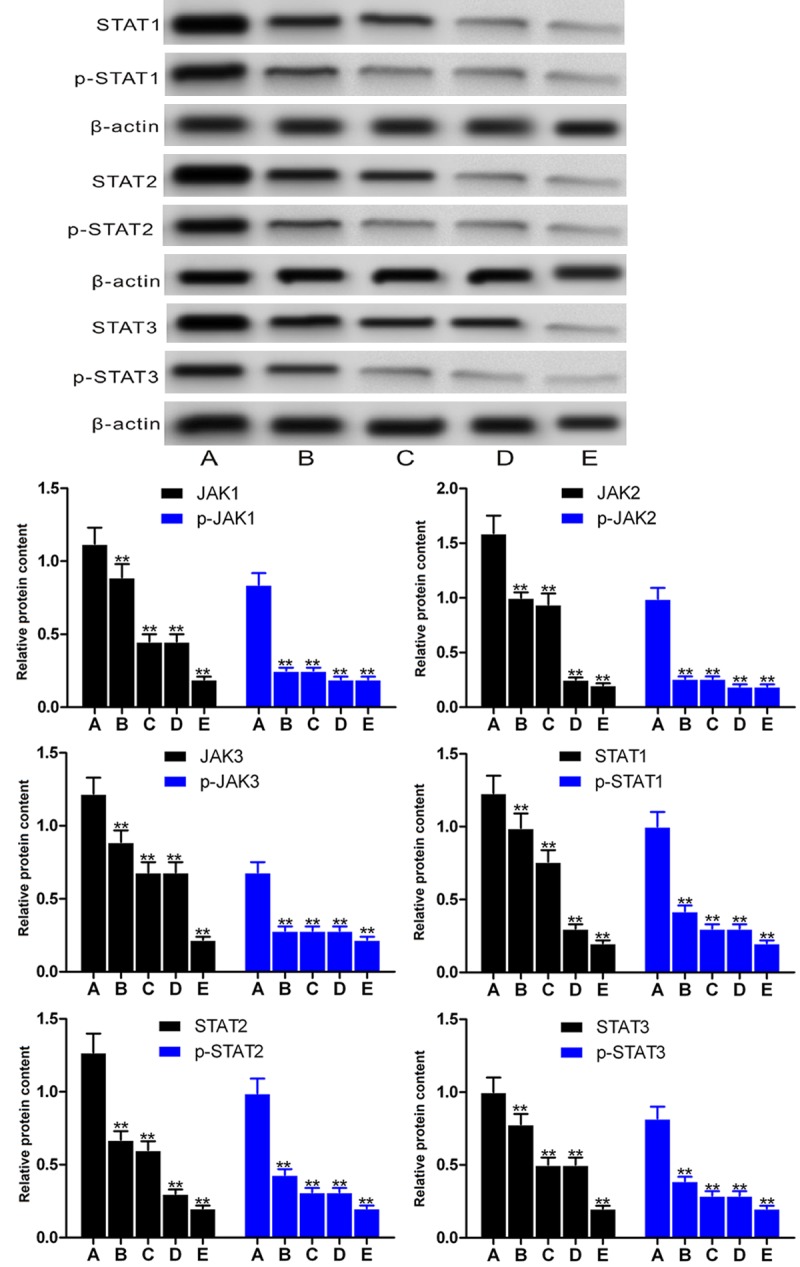
Levels of JAK/STAT signaling pathway proteins after treatment with MSC-CM and pathway inhibitors in the hypoxia/reoxygenation-treated HK-2 cells. A. Saline + HK-2 HRI (control); B. MSC-CM + HK-2 HRI; C. Group B + WP1066; D. Group B + SOCS and E, group B + WP1066 + SOCS. Upper pane, representative Western blots; lower pane, relative protein levels. **P < 0.01 vs untreated cells. **P < 0.01 vs saline-treated cells.
siRNA-STAT3 knockdowns the expression of STAT3, JAK2 and HMGB1 in HK-2 cells
When HK-2 cells were transfected with siRNA-STAT3, the levels of STAT3 mRNA and protein were significantly reduced (P < 0.01), while the level did not change when the cells were transfected with empty vector or not transfected (Figure 3A and 3B). Furthermore, qRT-PCR showed that levels of JAK2 and HMGB1 mRNA were also significantly reduced (P < 0.01, Figure 4A and 4B).
Figure 3.
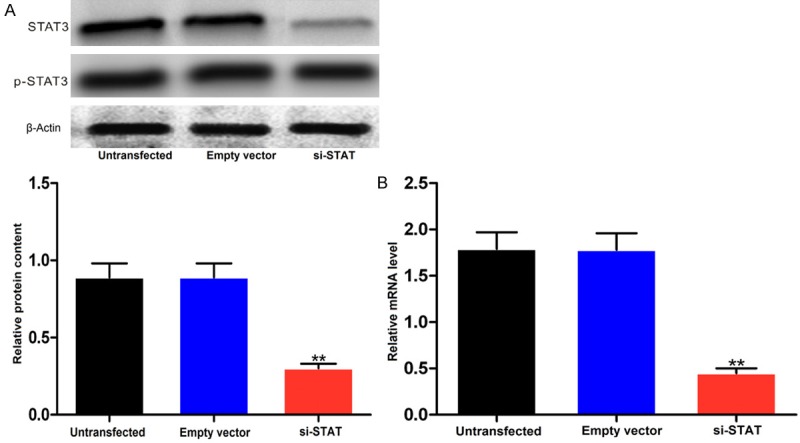
Protein (A) and mRNA (B) level of STAT3 in HK-2 cells transfected with siRNA-STAT3. Upper pane, representative Western blots; lower pane, relative protein levels. **P < 0.01 vs empty vector control.
Figure 4.
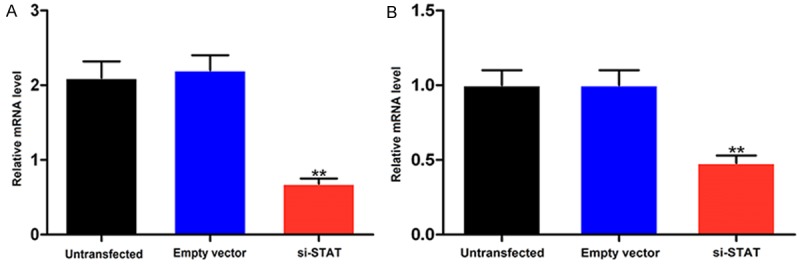
mRNA levels of JAK2 (A) and HMGB1 (B) in HK-2 cells transfected with siRNA-STAT3. **P < 0.01 vs empty vector control.
siRNA-STAT3 reduces cell viability and increases apoptosis in HK-2 cells
Finally, we examined the effect of STAT3 knockdown on viability and apoptosis in HK-2 cells. As shown in Figure 5A and 5B, the cell viability was significantly reduced (P < 0.01) and apoptosis significantly increased (P < 0.01) after the cells were transfected with siRNA-STAT3.
Figure 5.
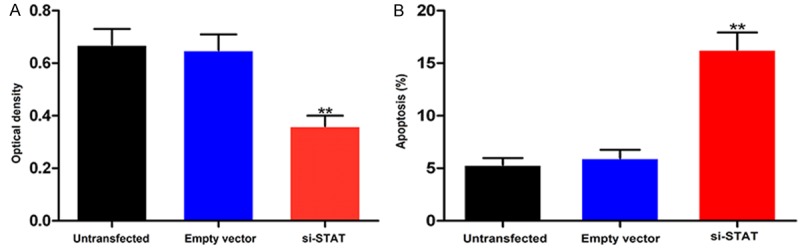
Viability (A) and apoptosis (B) of HK-2 cells after transfection with siRNA-STAT3. **P < 0.01 vs empty vector control.
Discussion
When exogenous MSCs are transplanted into the kidney IRI model, only less than 3% of them are able to reach the damaged sites in the kidney after being intercepted by lung, liver, spleen and other organs [2]. This process is highly dependent on the systemic or local inflammatory response caused by the renal injury. Renal IRI releases a variety of inflammatory factors associated with receptors expressed in MSCs. Gao et al. found that MSCs inhibit the JAK/STAT signaling pathway, suppress the renal IRI inflammatory response and promote the migration of MSCs to the damaged area [17]. The JAK/STAT signaling pathway is important for inflammatory factors and is activated when stimulated by upstream inflammatory factors such as interleukins and growth factors. The activated JAK receptor tyrosine kinase is phosphorylated to phosphorylate STATs to form dimers, which are then translocated to the nucleus to bind with nuclear DNA to regulate the transcription and translation of downstream inflammatory genes [18]. The JAK family has four kinases JAK1, JAK2, JAK3 and Tyk2. All of them have an active domain of tyrosine kinase. STATs are the target proteins of JAKs, including STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b and STAT6. Previous study confirmed that the phosphorylation levels of JAK2 and STAT3 are significantly increased after rat myocardial IRI, which produces a large number of inflammatory mediators, such as TNF-α and down-regulates Bcl-xl and up-regulates Bax expression. When given STAT3 inhibitor AG490, these changes are significantly inhibited, suggesting that JAK/STAT is closely related to cardiac IRI [8]. In addition, Wen et al. showed that in rat models subjected to superior mesenteric artery occlusion consisting of 60 min of ischemia and 2 h of reperfusion, the apoptotic index, cleaved caspase 3, phosphorylated JAK2, phosphorylated STAT1, and phosphorylated STAT3 expression were significantly enhanced, while subcutaneous administration of JAK2 inhibitor AG490 and STAT inhibitor significantly reduced the phosphorylated JAK2, phosphorylated STAT1, and phosphorylated STAT3, neutrophil aggregation and intestinal mucosal apoptosis, suggesting that JAK/STAT pathway activation plays a critical role in IRI-induced intestinal injury [9]. In addition, study also showed that the JAK/STAT signaling pathway is not only closely related to myocardium and intestinal IRI, but also to the renal IRI [19]. In this study, we first constructed a HRI model using HK-2 cells to simulate renal IRI, and found that in the HRI model, the levels of JAK/STAT signaling pathway proteins such as JAK1, JAK2, JAK3, p-JAK1, p-JAK2, p-JAK3, STAT1, STAT2, STAT3, p-STAT1, p-STAT2, and p-STAT3 were all up-regulated, suggesting that after renal IRI the JAK/STAT signaling pathway is activated. We then applied exogenous MSCs and JAK/STAT signaling pathway inhibitors to intervene. As a result, we found that exogenous MSCs, WP1066 and SOCS3 significantly inhibited the activation of the JAK/STAT signaling pathway, resulting in reduced expression of these pathway proteins, suggesting that exogenous MSCs can inhibit the activation of the JAK/STAT signaling pathway to reduce the HRI in HK-2 cells. This is consistent with early results [17].
STAT3 is the downstream target protein of JAK and the core member of the STATs family. After renal IRI, STAT3 and STAT5 phosphorylation levels are significantly increased [20]. To better understand and validate the role of the JAK/STAT signaling pathway in HRI-induced injury in HK-2 cells, we used a STAT3 knockdown vector to transfect HK-2 cells. Analysis showed that STAT3 mRNA and protein levels were significantly reduced, suggesting that the knockdown was successful. Furthermore, the expression of upstream signal molecule JAK2 was also reduced. This would allow to further deliberate the role of the JAK/STAT signaling pathway in HRI in HK-2 cells. In addition, we found that viability and apoptosis of HK-2 cells were reduced and increased, respectively, after they were transfected with siRNA-STAT3, suggesting that the JAK/STAT signaling pathway is associated with proliferation and apoptosis of HK-2 cells. Further study is needed to elucidate the detail relationship between the pathway and proliferation or apoptosis of HK-2 cells in HRI models.
HMFB1 is the most important mediators in renal IRI inflammatory response [6]. Lu et al. confirmed that the JAK/STAT signaling pathway can regulate the release of HMGB1 induced by LPS and IFN-β [11]. Liu et al. found that LPS can stimulate the expression of HMGB1 in macrophages to activate the JAK/STAT signaling pathway, while JAK2 inhibitor AG490 and STAT1 inhibitor fludarabine added to LPS-induced macrophages can significantly down-regulate HMGB1 expression and inactivate the JAK/STAT signal pathway. When macrophages were further treated with 10 µg/mL HMGB1, they found that that JAK/STAT signaling pathway is activated and TNF-β expression is increased, while the JAK/STAT signaling pathway inhibitors can reverse the changes, suggesting that HMGB1 can modulate inflammatory response via the JAK/STAT signaling pathway [10]. We found that HMGB1 expression was significantly down-regulated in siRNA-STAT3 transfected HK-2 cells, suggesting that the inactivation of JAK/STAT signaling pathway reduces the expression level of HMGB1 to modulate the inflammatory reaction. HMBG is a class of highly conserved non-histone DNA binding proteins, consisting of HMGB1, HMGB2 and HMGB3. HMGB1 is widely distributed in heart, liver, spleen, lung, kidney and brain tissues, and mainly exists in the nucleus. It is involved in DNA repair and recombination. HMGB1 as an important inflammatory mediator in the IRI of kidney and other tissues is induced by TNF-α, IL-1β and IL-6, which in turn stimulates the production of IFN-γ and TNF-α [21]. Studies have demonstrated that HMGB1 may play an important role in lupus nephritis, acute kidney injury, interstitial nephritis and IRI. For example, Li et al. found that its expression is low in normal mouse renal parenchymal cells, but increased after renal ischemia for 1 h and reached the maximum after reperfusion for 3 h [5]. Several other studies also show that the HMGB1 can neutralize antibody to induce renal IRI and inhibit the expression of inflammatory factors such as MCP1 [22-24], suggesting that by inhibiting the expression of HMGB1 in the kidney after IRI, it may be possible to reduce the IRI-induced inflammatory reactions to a certain extent, thereby preventing further deterioration of renal IRI. Our study also confirms that suppressing JAK/STAT signaling pathway results in reduced HMGB1 expression, indicating that this mechanism may be involved in intervening renal IRI inflammatory response.
Conclusion
Our findings demonstrate that exogenous MSCs down-regulate the expression of HMGB1 through inhibiting the JAK/STAT signaling pathway, resulting in reduced HRI in HK-2 cells.
Acknowledgements
The work was supported by Guangdong Province Science and Technology Department (grant nos. 2014A020211027 and 2015B020226002) and Scientific Research Projects of Universities administered by Guangzhou (grant no. 1201630620).
Disclosure of conflict of interest
None.
References
- 1.Zhang H, Zhang T. Research progress of the mechanisms of stem cells improving renal ischemia-reperfusion injury. Medical Recapitulate. 2014;20:3114–3116. [Google Scholar]
- 2.Tao Y, Ye L, Wang Y, Wang Z. Mechanism for promoting repair of renal ischemia reperfusion injury by mesenchymal stem cells. Chin J Contemp Pediatr. 2013;15:157–160. [PubMed] [Google Scholar]
- 3.Salvadori M, Rosso G, Bertoni E. Update on ischemia-reperfusion injury in kidney transplantation: pathogenesis and treatment. World J Transplant. 2015;5:52–67. doi: 10.5500/wjt.v5.i2.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang L, Chen Z, Xie S, Ma J, Fang J, Li G, Xu L, Zhang Y, Guo Y, Pan G. Safety and feasibility of autologous bone marrow mesenchymal stem cells in treating chronic allograft nephropathy. Chinese Journal of Tissue Engineering Research. 2014;18:5140–5145. [Google Scholar]
- 5.Li J, Gong Q, Zhong S, Wang L, Guo H, Xiang Y, Ichim TE, Wang CY, Chen S, Gong F, Chen G. Neutralization of the extracellular HMGB1 released by ischaemic damaged renal cells protects against renal ischaemia-reperfusion injury. Nephrol Dial Transplant. 2011;26:469–478. doi: 10.1093/ndt/gfq466. [DOI] [PubMed] [Google Scholar]
- 6.Han R, Ye Z. High mobility group box 1 protein and kidney diseases. J Nephrol Dialy Transplant. 2013;22:262–267. [Google Scholar]
- 7.Feng X, Li X, Wang X. Effect of JAK/STAT pathway on renal ischemia-reperfusion injury in rats. J Chin PLA. 2011;36:218–220. [Google Scholar]
- 8.Ottani A, Galantucci M, Ardimento E, Neri L, Canalini F, Calevro A, Zaffe D, Novellino E, Grieco P, Giuliani D, Guarini S. Modulation of the JAK/ERK/STAT signaling in melanocortin-induced inhibition of local and systemic responses to myocardial ischemia/reperfusion. Pharmacol Res. 2013;72:1–8. doi: 10.1016/j.phrs.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Wen SH, Li Y, Li C, Xia ZQ, Liu WF, Zhang XY, Lei WL, Huang WQ, Liu KX. Ischemic postconditioning during reperfusion attenuates intestinal injury and mucosal cell apoptosis by inhibiting JAK/STAT signaling activation. Shock. 2012;38:411–419. doi: 10.1097/SHK.0b013e3182662266. [DOI] [PubMed] [Google Scholar]
- 10.Liu H, Yao YM, Yu Y, Dong N, Yin HN, Sheng ZY. Role of Janus kinase/signal transducer and activator of transcription pathway in regulation of expression and inflammation-promoting activity of high mobility group box protein 1 in rat peritoneal macrophages. Shock. 2007;27:55–60. doi: 10.1097/01.shk.0000233197.40989.31. [DOI] [PubMed] [Google Scholar]
- 11.Lu B, Antoine DJ, Kwan K, Lundback P, Wahamaa H, Schierbeck H, Robinson M, Van Zoelen MA, Yang H, Li J, Erlandsson-Harris H, Chavan SS, Wang H, Andersson U, Tracey KJ. JAK/STAT1 signaling promotes HMGB1 hyperacetylation and nuclear translocation. Proc Natl Acad Sci U S A. 2014;111:3068–3073. doi: 10.1073/pnas.1316925111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azizi SA, Stokes D, Augelli BJ, DiGirolamo C, Prockop DJ. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats--similarities to astrocyte grafts. Proc Natl Acad Sci U S A. 1998;95:3908–3913. doi: 10.1073/pnas.95.7.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Zhang X, Tang W, Zheng L. Primary culture conditions of rat bone marrow mesenchymal stem cells. Journal of Clinical Rehabilitative Tissue Engineering Research. 2009;13:2740–2745. [Google Scholar]
- 14.Yan YF, Yang WJ, Xu Q, Chen HP, Huang XS, Qiu LY, Liao ZP, Huang QR. DJ-1 upregulates anti-oxidant enzymes and attenuates hypoxia/re-oxygenation-induced oxidative stress by activation of the nuclear factor erythroid 2-like 2 signaling pathway. Mol Med Rep. 2015;12:4734–4742. doi: 10.3892/mmr.2015.3947. [DOI] [PubMed] [Google Scholar]
- 15.Sun Y, Guo BF, Xu LB, Zhong JT, Liu ZW, Liang H, Wen NY, Yun WJ, Zhang L, Zhao XJ. Stat3-siRNA inhibits the growth of gastric cancer in vitro and in vivo. Cell Biochem Funct. 2015;33:495–502. doi: 10.1002/cbf.3148. [DOI] [PubMed] [Google Scholar]
- 16.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 17.Gao H, Priebe W, Glod J, Banerjee D. Activation of signal transducers and activators of transcription 3 and focal adhesion kinase by stromal cell-derived factor 1 is required for migration of human mesenchymal stem cells in response to tumor cell-conditioned medium. Stem Cells. 2009;27:857–865. doi: 10.1002/stem.23. [DOI] [PubMed] [Google Scholar]
- 18.Xue X, Xu H, Shao D, Tang W, Nie H. Research progress of regulating mechanism on JAK/STAT signaling pathway. Progress in Modern Biomedicine. 2015;15:2161–2164. [Google Scholar]
- 19.Correa-Costa M, Azevedo H, Amano MT, Goncalves GM, Hyane MI, Cenedeze MA, Renesto PG, Pacheco-Silva A, Moreira-Filho CA, Camara NO. Transcriptome analysis of renal ischemia/reperfusion injury and its modulation by ischemic pre-conditioning or hemin treatment. PLoS One. 2012;7:e49569. doi: 10.1371/journal.pone.0049569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huen SC, Huynh L, Marlier A, Lee Y, Moeckel GW, Cantley LG. GM-CSF promotes macrophage alternative activation after renal ischemia/reperfusion injury. J Am Soc Nephrol. 2015;26:1334–1345. doi: 10.1681/ASN.2014060612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han F. HMGB1: extracellular release and signal transduction mechanisms. J Med Postgra. 2013;26:296–299. [Google Scholar]
- 22.Wu H, Ma J, Wang P, Corpuz TM, Panchapakesan U, Wyburn KR, Chadban SJ. HMGB1 contributes to kidney ischemia reperfusion injury. J Am Soc Nephrol. 2010;21:1878–1890. doi: 10.1681/ASN.2009101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu H, Steenstra R, de Boer EC, Zhao CY, Ma J, van der Stelt JM, Chadban SJ. Preconditioning with recombinant high-mobility group box 1 protein protects the kidney against ischemia-reperfusion injury in mice. Kidney Int. 2014;85:824–832. doi: 10.1038/ki.2013.475. [DOI] [PubMed] [Google Scholar]
- 24.Ruan Y, Wang L, Zhao Y, Yao Y, Chen S, Li J, Guo H, Ming C, Chen S, Gong F, Chen G. Carbon monoxide potently prevents ischemia-induced high-mobility group box 1 translocation and release and protects against lethal renal ischemia-reperfusion injury. Kidney Int. 2014;86:525–537. doi: 10.1038/ki.2014.80. [DOI] [PubMed] [Google Scholar]


