Abstract
Transmembrane protein 100 (TMEM100) was first identified as a transcript from the mouse genome. Recent studies have demonstrated that TMEM100 is involved in hepatocellular carcinoma (HCC) malignancy. However, the distribution and clinical significance of TMEM100 in non-small-cell lung carcinoma (NSCLC) remains poorly understood. This study aims to explore the significance of TMEM100 expression in NSCLC. We found that TMEM100 expression was significantly reduced in NSCLC tissues when compared with that in adjacent normal lung tissues (P<0.001). Kaplan-Meier survival analysis showed that overall survival of patients with lower expressions of TMEM100 was significantly shorter (n=152, P<0.05). In addition, TMEM100 overexpression in NSCLC cell lines inhibited cell proliferation in vitro and in vivo. Transwell migration and invasion assay showed that TMEM100 significantly suppressed the migration and invasion of NSCLC cell lines. In contrast, knocking down TMEM100 promoted NSCLC proliferation and migration. Finally, we found that TMEM100 worked as a cancer suppressor gene mainly by inhibiting the TNF signaling pathway. In conclusion, TMEM100 acted as a tumor suppressor in NSCLC and may prove to be a potential prognostic biomarker and therapeutic target for NSCLC.
Keywords: TMEM100, non-small-cell lung cancer (NSCLC), tumor necrosis factor (TNF), prognosis, signaling pathway
Introduction
Lung cancer is one of the most common cancers and the leading cause of cancer-related death worldwide, accounting for about 14% of all new cancer diagnoses, with estimated 222,200 new cases in 2015 [1-3]. Non-small-cell lung carcinoma (NSCLC), mainly including lung adenocarcinoma and squamous cell lung carcinoma, accounts for approximately 80% of all lung cancers [4]. Despite remarkable improvements in the treatment of NSCLC during recent decades, the mortality rate remains high mainly due to delayed diagnosis and the lack of effective therapies [5]. In recent decades, various molecules such as miR-338-3p, miR-296-3p, GOLPH3, CREPT and HOTTIP have been reported to play some role in invasion and migration of NSCLC [6-10]. Although these findings represent significant progress in this field, the mechanisms underlying NSCLC metastasis remain largely unknown. Therefore, it is very important to identify novel tumor suppressor-genes to prevent the recurrence and metastasis of NSCLC.
Transmembrane protein 100 (TMEM100) was first identified as a transcript from the mouse genome. It is known to be located at 17q32 encoding a 134-amino acid protein with two hypothetical transmembrane domains (amino acids 53-75, 85-107) [11,12]. TMEM100 is highly conserved in two invertebrate species and not structurally related to any known family of proteins in any species [12]. A recent study [13] reported that TMEM100 was detectable in blood vessels of developing organs but diminished in the adult stage except in the lung [13]. Frullanti et al [14] found that TMEM100 was related to the clinical stage of lung adenocarcinoma, and inhibited the clonogenicity of H460 and H520 cells. However, its expression and roles in NSCLC remain unclear.
The purpose of the present study was to investigate the expression and roles of TMEM100 in NSCLC, and explore the underlying mechanism and relationship between TMEM100 expression and the prognosis of NSCLC patients. The results showed that tumor necrosis factor (TNF) was a target gene of TMEM100, and TMEM100 exerted an inhibitory effect on NSCLC cells by suppressing the TNF signaling pathway.
Materials and methods
Tissue specimens
Specimens of 152 lung cancer patients and 78 normal lung tissues were obtained from patients who underwent lung resection at Changzheng Hospital of the Second Military Medical University (Shanghai, China) between January 2010 and October 2015. The study was undertaken with the understanding and written consent of each patient. None of the patients in our study received neoadjuvant chemotherapy. The histological differentiation and histological type of these samples were assessed by the experienced pancreatic pathologists. Clinical data of the patients including age, sex, tumor size, stage, pathology grade and the expression level of TMEM100 are summarized in Table 1.
Table 1.
Patient characteristics (n=152)
| Characteristics | Values |
|---|---|
| Age (years) | 59.9±9.7 |
| Sex (male/female) | 110/42 |
| Types (AD/SCC) | 78/74 |
| Size (cm) | 4.8±1.6 |
| Pathology grade (I/II/III) | 17/95/40 |
| T grade (1/2/3) | 13/119/20 |
| N grade (0/1/2) | 95/45/12 |
| AJCC grade (1/2/3) | 50/84/18 |
| TMEM100 (negative/low/high) | 35/81/36 |
Immunohistochemistry
Tumor tissues or other samples were fixed with 4% paraformaldehyde and then dehydrated through a graded series of ethanol, paraffin embedded, and sliced into 5-μm sections. Immunohistochemical staining for TMEM100 (aya36508, ayabio, China) was carried out using standard histological procedures described in the manual for Histostain-Plus (DAB) kit (Mingrui Biotech). TMEM100 staining was scored in accordance by two investigators with previous protocols as negative, low or high. Scoring was performed blindly with respect to the histologic grade of the lung cancer specimens.
Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated by using TRIZOL (Invitrogen, USA) and reverse transcribed into cDNA by using the Prime Script™ RT Master Mix (Takara, Japan). Gene transcripts were quantified on 7900HT Fast Real-Time PCR System (Life Technologies Corporation, USA) using SYBR® Premix Ex Taq™ II (Takara, Japan) and normalized with GAPDH. All primers are listed in Supplementary Table 1.
TMEM100 overexpression plasmid and siRNA
pcDNA3.1+ plasmid was enzyme digested by KpnI and EcoRV (TransGen Biotech, China). The code sequence of TMEM100 was amplified by PCR method and inserted into pcDNA3.1+ by using the Quick-Fusion Cloning Kit (biotool, USA). The PCR primer sequence was: S: TTA AAC TTA AGC TTG GTA CAT GAC TGA AGA GCC CAT CAA G; A: GGC CGC CAC TGT GCT GGA TCA AGC AAA CAA GCT TCT CTG. The TMEM100 overexpression (OE) plasmid was validated by DNA sequencing. Two different siRNA oligos against TMEM100 were purchased from IDT. siRNA-1: 5-CAG ACU UUA UGU UCA UAG UUC UUC CUC-3; siRNA-2: 5-CUU CCA CAA CUA CAU AGG GUA UUG UUU-3. RT-PCR and Western blot assay were used to confirm the expression of TMEM100 after transfection.
Cell culture and transfection
Two NSCLC cell lines A549 and PC9 were purchased from the American Type Culture Collection (ATCC, USA), and routinely maintained in DMEM and RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA), 100 U/ml penicillin sodium, and 100 mg/ml streptomycin sulfate at 37°C in a humidified air atmosphere containing 5% CO2. Cells were used when they were in the logarithmic growth phase. Cells were transfected using DNA Transfection Reagent (biotool, USA) when they grew to 50~70% confluence according to the manufacturer’s instructions.
Western blot
Total protein (100 ug) was separated using SDS-PAGE and then transferred onto nitrocellulose membranes (Thermo, USA). The blotted membranes were incubated with TMEM100 antibody (aya36508, ayabio, China) diluted at 1:1000. After washing, the membranes were incubated with a 1:5000 dilution of horseradish peroxidase-linked goat anti- rabbit and goat anti- mouse antibodies (Santa Cruz, USA). The blots were developed using EasySee Western Blot Kit (TransGen Biotech, China). Beta-actin protein was also determined by using the specific antibody (Santa Cruz, USA) as a loading control. All experiments were carried out in triplicate.
CCK8 assay
Cells were digested, counted, seeded in 96-well plates, cultured for 0, 12, 24, 48 and 72 h, and finally assessed using the Cell Counting Kit 8 (biotool, USA). The results were measured by absorbance at 450 nm using an ELx800 microplate reader (BioTek Instruments Inc., USA).
Cell cycle assay
For cell cycle assay, A549 cell and PC9 were seeded in 6-well plates and transfected with OE-TMEM100 or pcDNA3.1+ plasmids for 48 h. Cells were collected and fixed with ice-cold 70% ethanol, washed with PBS, and resuspended in 0.5 mL PBS containing propidium iodide (0.5 mg/mL in PBS with 0.1% sodium azide) and RNase A (1 mg/mL). After final incubation at 37°C for 30 min, cells were analyzed using a FACSCalibur flow cytometer (Becton-Dickinson, USA). Data were analyzed using FlowJo software (Tree Star, USA).
Transwell migration and invasion assay
An 8-μm pore size transwell chamber without matrigel (3422, Corning, USA) was used for transwell migration assay, and an 8-μm pore size transwell chamber with matrigel (354480, Corning, USA) was used for transwell invasion assay. Cells were digested and counted. A total of 1 × 105 cells in 100 μL medium supplemented with no FBS were plated in the upper chamber and 500 μL medium supplemented with 10% FBS was covered on the bottom chambers as chemoattractant. After 24-h incubation in a humidified incubator, non-migratory cells on the upper membrane surface were carefully removed, and those on the bottom surface were fixed with 4% polyoxymethylene and stained with 0.1% crystal violet for 15 min. Cells were counted by photographing 5 random fields under a microscope at 400 × magnification. The acetic acid elution was next done and the absorbance was recorded at 570 nm.
Illumina mRNA deep sequencing
PC9 cells were harvested after being transfected with OE-TMEM100 or pcDNA3.1+ plasmids for 48 h. Total RNA was extracted from the cells using TRIZOL Reagent according the manufacturer’s instructions (Invitrogen, USA), and subjected to Illumina mRNA deep sequencing using a service from LC Sciences (Houston, TX, USA). The sequence results were obtained as the FPKM (fragment per kilobase of exons per million reads) for each transcript.
The mouse model
We first constructed cell lines by using PC9 cells infected with a control vector or TMEM100 lentivirus. Then, 5 × 106 PC9 cells were injected subcutaneously into the left upper flank region of the nude mouse (3-4 weeks of age, male, BALB/c). The subcutaneous tumor tissue was removed in a month. The tumor size was calculated as follows: tumor volume (mm3) = (L × W2)/2, where L = long axis and W = short axis.
Statistical analysis
SPSS 19.0 statistical software (SPSS Inc., Chicago, IL) was used for statistical analysis. All data are presented as mean ± standard error of the mean (SEM). The survival probability was estimated by the Kaplan-Meier methods, and Log-rank tests were used to compare overall survival (OS) between groups. Factors of P≤0.1 in Log-rank tests were subjected to the Cox proportional hazards analysis and calculation of the hazard ratio and 95% confidence interval (CI). Statistics of the mean value between groups were assessed using both Student t test and ANOVA, assuming double-sided independent variance. All experiments were repeated at least three times, and representative experiments are shown. P values of <0.05 were considered statistically significant.
Results
Down-regulation of TMEM100 is associated with NSCLC poor prognosis
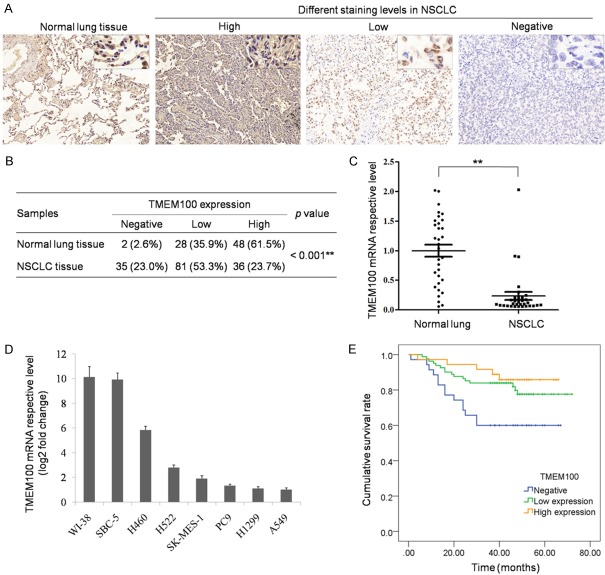
In this study, we detected the expression of TMEM100 in 152 primary NSCLC and 78 normal lung tissues by immunohistochemistry staining (Figure 1A). Univariate and multivariate analyses were used to analyze the correlation between the clinical data and TMEM100 expression (Table 2). The results showed that TMEM100 was mainly expressed in the cytomembrane, and significantly downregulated in NSCLC tissues as compared with that in normal lung tissues (Figure 1B). Then, the expression of TMEM100 mRNA was verified by RT-PCR in 32 NSCLC tissues and para-tumor normal tissues (Figure 1C and 1D). The result was consistent with the result of immunohistochemical staining (P<0.001). Furthermore, the results showed that the expression level of TMEM100 was positively correlated with disease prognosis. NSCLC patients with negative TMEM100 usually had poor diagnosis (Figure 1E and Supplementary Figure 1).
Figure 1.
TMEM100 was down-regulated in NSCLC and associated with poor prognosis. A. Detection of TMEM100 expression in normal lung tissue and NSCLC tissue by immunohistochemical staining of TMEM100, as expressed as negative, low or high. B. Statistics of all samples associated with TMEM100 staining. C. Verification of TMEM100 expression in normal lung tissue and NSCLC tissue by PCR. D. Detection of TMEM100 expression in different NSCLC cells by qPCR. E. Analysis of the association between TMEM100 expression and overall survival of 152 NSCLC patients by Kaplan-Meier survival Curves. *: P<0.05; **: P<0.001.
Table 2.
Analysis of different prognostic factors in 152 NSCLC patients
| Prognostic factors | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age (≤60/>60) | 1.626 | 0.833-3.177 | 0.155 | |||
| Sex (Male/Female) | 0.402 | 0.156-1.035 | 0.059 | 0.414 | 0.158-1.084 | 0.072 |
| Types (AD/SCC) | 1.446 | 0.740-2.824 | 0.281 | |||
| Size (≤5 cm/>5 cm) | 2.142 | 1.103-4.157 | 0.024* | 1.252 | 0.580-2.700 | 0.567 |
| Pathology grade (I-II/III) | 1.569 | 0.780-3.155 | 0.206 | |||
| T grade (1-2/3) | 2.903 | 1.359-6.205 | 0.006* | 2.275 | 1.049-4.933 | 0.037* |
| N grade (0/1-2) | 2.042 | 1.101-3.786 | 0.023* | 2.248 | 1.195-4.228 | 0.012* |
| AJCC grade (1/2-3) | 2.149 | 0.939-4.921 | 0.070 | 1.044 | 0.353-3.087 | 0.939 |
| TMEM100 (high/negative) | 3.525 | 1.268-9.797 | 0.016* | 3.445 | 1.231-9.642 | 0.018* |
| TMEM100 (high/low) | 1.496 | 0.548-4.083 | 0.432 | 1.294 | 0.469-3.567 | 0.619 |
P<0.05.
TMEM100 overexpression inhibits NSCLC cell proliferation in vitro and in vivo
To detect the function of TMEM100 in lung cancer cells, we constructed the overexpression plasmid TMEM100-pcDNA3.1+, and then transfected TMEM100-pcDNA3.1+ in A549 and PC9 cells. The transfection efficacy was verified by PCR and western-blot (Figure 2A and 2B). After successful overexpression of TMEM100 protein in A549 and PC9 cells, MTT analysis was performed to detect cell proliferation. The result showed that TMEM100 overexpression inhibited A549 and PC9 cell proliferation (Figure 2C and 2D), and this inhibitory effect became more obvious with the prolonging of time (P<0.001). Furthermore, cell cycle analysis by flow cytometry showed that TMEM100 overexpression suppressed A549 and PC9 cell cycle, which was consistent with the result of MTT analysis (Figure 2E and 2F). The percentage of cells in G1-phase was increased and the percentage of G2-M phase cells was decreased.
Figure 2.
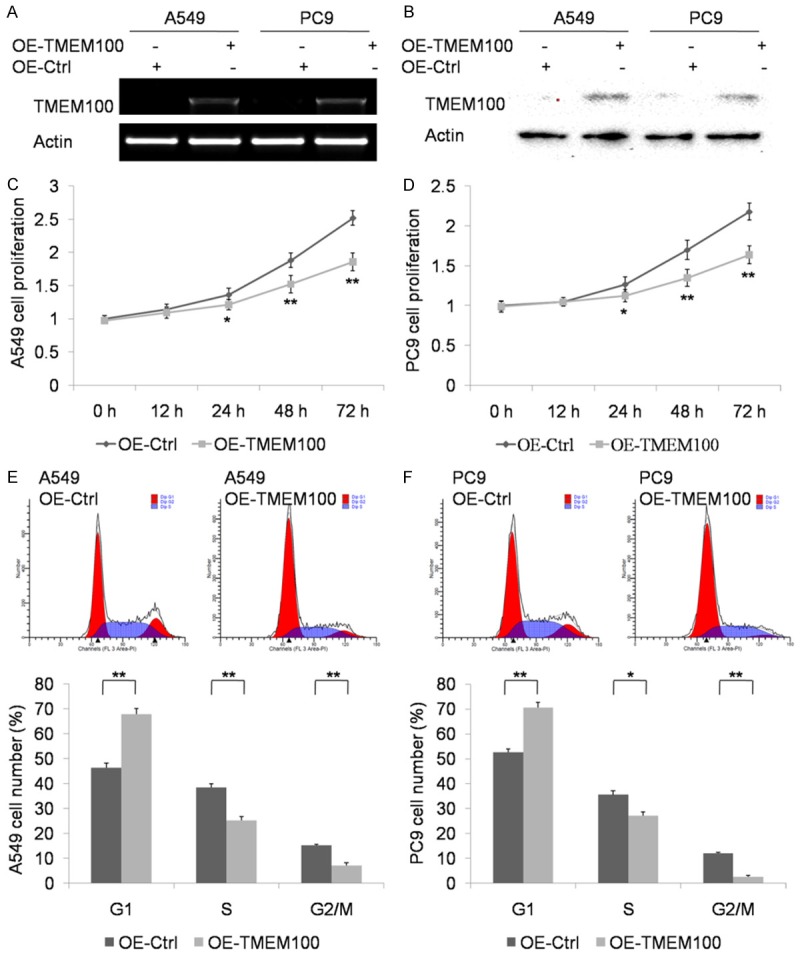
TMEM100 overexpression inhibits NSCLC cell proliferation in vitro. A, B. Confirmation of TMEM100 overexpression after transfection with OE-TMEM100 or pcDNA3.1+ plasmids by PCR and Western blot. C, D. Detection of the proliferation of A549 and PC9 cells transfected with OE-TMEM100 or pcDNA3.1+ plasmids by CCK8 assay. E, F. Cell cycle assay for cell cycle of A549 and PC9 cells transfected with OE-TMEM100 or pcDNA3.1+ plasmids. *: P<0.05; **: P<0.001.
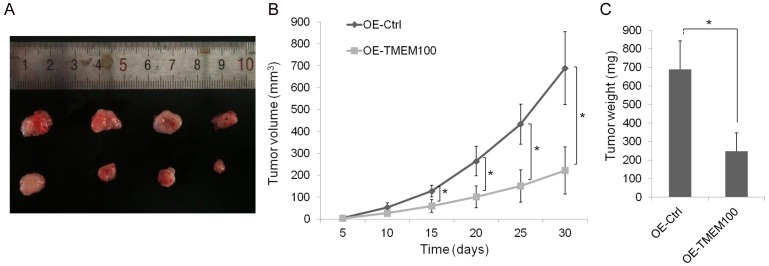
To construct a stable cell line, PC9 cells were infected with the control vector or TMEM100 lentivirus. RT-PCR and Western blot assays were used to confirm the expression of TMEM100 after transfection (Supplementary Figure 2). The effect of TMEM100 expression on tumor cells in vivo was also examined (Figure 3A-C). The result showed that the tumor size in TMEM100 overexpression group was significantly smaller than that in the control group (P<0.05).
Figure 3.
TMEM100 overexpression inhibits NSCLC cell proliferation in vivo. The model in nude mice was constructed by using PC9 cells infected with control vector or TMEM100 lentivirus. A. The size of subcutaneous tumors in these two groups were calculated and compared. B, C. The size and weight of tumors in overexpressed TMEM100 group was significantly smaller than that of control group (P<0.05). *: P<0.05; **: P<0.001.
TMEM100 overexpression inhibits NSCLC cell migration and invasion
Knowing that TMEM100 overexpression inhibited NSCLC cell proliferation and suppressed cell cycle, we further explored its potential effect on NSCLC cell migration and invasion by Transwell invasion and migration assays. The result showed that the number of A549 and PC9 cells migrating through the chamber membrane in TMEM100-pcDNA3.1+ transfection group was decreased significantly as compared with that in the blank control group (Figure 4A). The invasion capability associated with TMEM100 expression was examined with Transwell chambers coated with matrigel. As expected, the number of cells migrating through the membrane was reduced markedly when TMEM100 was overexpressed (Figure 4B). These results demonstrated that TMEM100 overexpression suppressed NSCLC cell migration and invasion.
Figure 4.
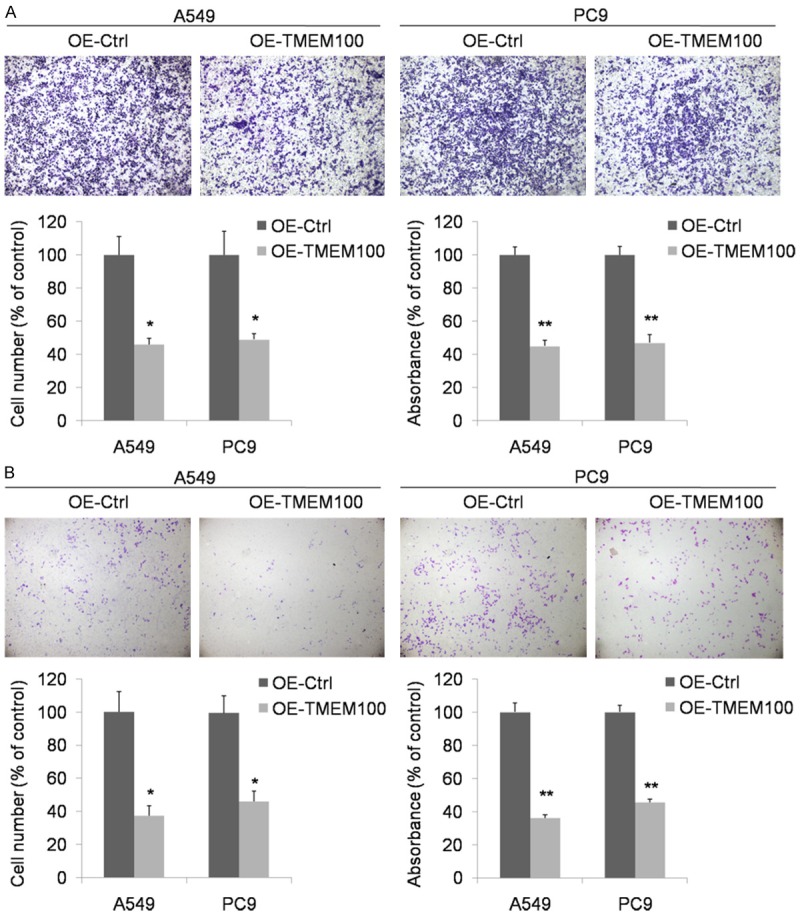
Transwell migration and invasion assay proved that TMEM100 overexpression inhibited the migration and invasion abilities of A549 and PC9 cells. A. Transwell migration assay showed that the number of cells migrating through the membrane was decreased markedly when TMEM100 was overexpressed. B. Transwell invasion assay showed that the number of cells migrating through the membrane was decreased markedly when TMEM100 was overexpressed. *: P<0.05; **: P<0.001.
TMEM100 knockdown promotes NSCLC cell proliferation and migration
The H522 cell line highly expressing TMEM100 was selected as the model in this experiment. Two types of siRNA were transfected in H522 cells. RT-qPCR and Western-blot were performed to confirm the effect of siRNA. As shown in Figure 5A and 5B, the expression of TMEM100 was reduced markedly siRNA transfection. Then, cell proliferation was tested by CCK8 (Figure 5C). It was found that the cell proliferation ability was promoted by TMEM100 knockdown. In addition, Transwell assay showed that TMEM100 knockdown also enhanced H522 cell migration (Figure 5D). These data support that TMEM100 played an important role in inhibiting NSCLC metastasis and proliferation.
Figure 5.
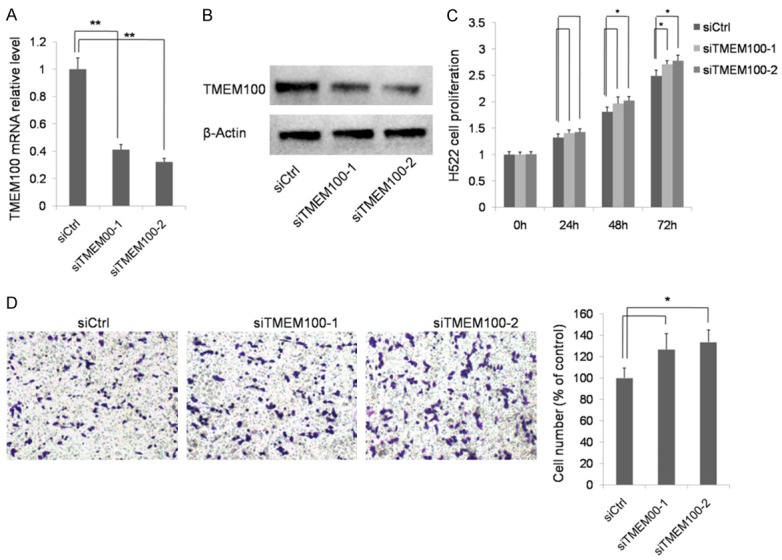
TMEM100 knockdown promotes NSCLC cell proliferation and migration. A, B. Confirmation of TMEM100 knockdown after transfection with siRNA by PCR and Western blot. C. Detection of the proliferation of H522 cells transfected with siCtrl, siTMEM100-1 or siTMEM100-2 by CCK8 assay. D. Transwell invasion assay showed that the number of cells migrating through the membrane was increased markedly when TMEM100 was knockdown. *: P<0.05; **: P<0.001.
The gene expression profile of PC9 cells in TMEM100 overexpression vscontrol groups
Microarray analysis of total RNA from PC9 cells in TMEM100 overexpression vs. control groups revealed statistically significant differences in expression levels between the two transcripts (Figure 6A and 6B). As shown in Figure 4B, numerous genes involved in tumor necrosis factor (TNF) signaling pathways were downregulated by more than two fold in TMEM100 overexpression group. They typically included TNF, TRADD, FOS, JUN, BCL3, EDN1 and CSF1. This result was further verified by RT-PCR, showing that there were significant differences in the expression of all the 7 candidate genes in A549 cells between TMEM100 overexpression and control groups (P<0.05) (Figure 6C-I). Pathway and GO assays indicated that TMEM100 participated in TNF pathway (Supplementary Figure 3). These results strongly suggest that TMEM100 may function as a tumor suppressor by inhibiting TNF pathway.
Figure 6.
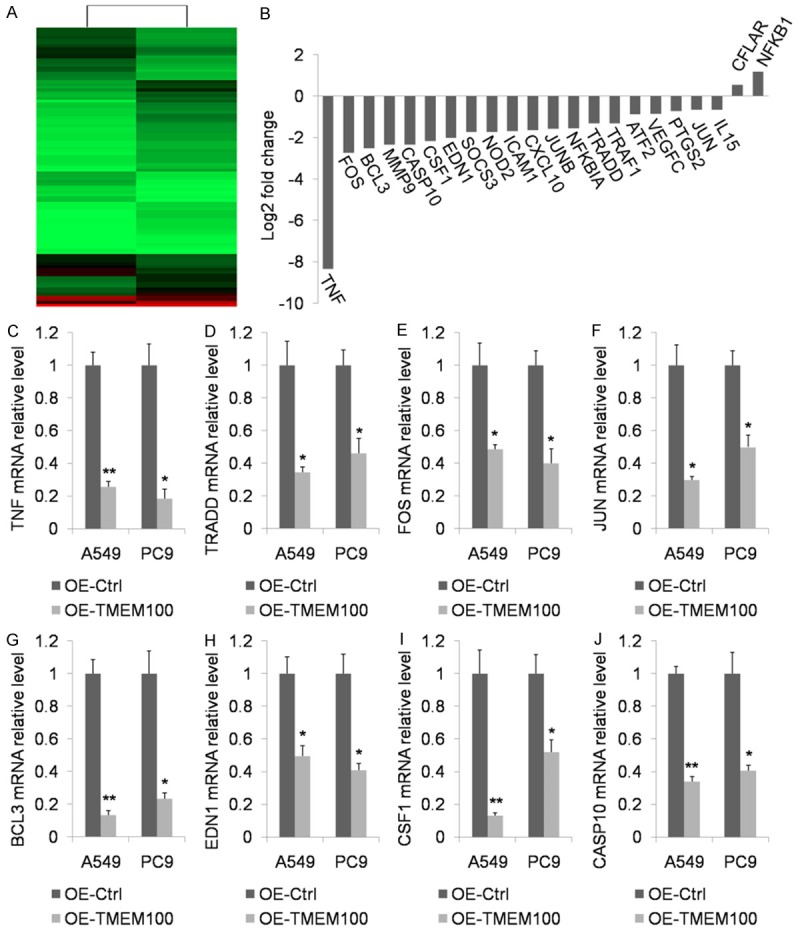
mRNA deep sequencing was applied to examine the difference in gene expression profiles after PC9 cell transfection with OE-TMEM100 or pcDNA3.1+ plasmids. A. A heat map showing that the expression level of some transcripts differed significantly between PC9 cells transfected with OE-TMEM100 and those with pcDNA3.1+ plasmids. B. The expression level of genes participating in TNF singling pathway underwent significant changes. C-I. qPCR was performed to verify genes participating in TNF singling pathway. J. Western blot assay was used to confirm the overexpression of TNF after OE-TMEM100 or pcDNA3.1+ plasmid transfection. *: P<0.05; **: P<0.001.
Discussion
NSCLC is among the leading causes of cancer related death. Despite remarkable progress in the screening, diagnosis and clinical management of lung cancer, the outlook for patients with NSCLC remains dismal, with only slightly more than 15% patients living longer than 5 years after diagnosis [5]. Although our understanding about the pathogenesis of NSCLC has improved through identification of plentiful molecules, the mechanism underlying NSCLC progression, including cell proliferation, migration and metastasis remains elusive. Therefore, it is very important to identify novel tumor suppressor-genes to prevent the disease progression.
TMEM100 is a novel gene firstly identified as a transcript from the mouse genome in 2001. TMEM100 is known as a potentiating modulator of TRPA1-V1 complexes that contributes to persistent pain [15]. Eisenman et al [16] identified TMEM100 as a novel membrane-associated marker for enteric nerves and is as effective as protein gene product 9.5 for identifying neuronal structures in the gastrointestinal tract. The expression of TMEM100 in the enteric nervous system may reflect a role in the development and differentiation of cells through a transforming growth factor β, BMP or related signaling pathway. In the lungs of ALK1-deficient mice, TMEM100 expression was found to be downregulated, implying that it was involved in the ALK1/transforming growth factor beta (TGF-β) signaling pathway [17]. More recent studies [18-20] demonstrated that TMEM100 was involved in apoptosis, angiogenesis and body height. One study [21] even detected elevation of TMEM100 in the transition zone of the prostate as compared with that in the peripheral zone where most aggressive tumors are detected, suggesting that it may be a potent tumor suppressor candidate. It was also found [22] that TMEM100 was an independent risk factor for overall survival and disease-free survival in hepatocellular carcinoma (HCC) patients, suggesting that TMEM100 may function as a tumor suppressor in HCC metastasis and proliferation.
However, the role of TMEM100 in human NSCLC remains unknown, and the correlation between its expression and prognosis of NSCLC patients has not been documented. Thus, we firstly measured TMEM100 expression in 178 NSCLC tissue specimens and 52 normal lung tissue specimens by Immunohistochemistry. It was found that TMEM100 was frequently downregulated in NSCLC tumor tissues compared with normal lung tissues. According to the data of immunohistochemistry, we divided all patients into three groups: high, low and negative. Generally, NSCLC patients in high TMEM100 expression group had better prognosis than those in the negative group. To confirm the expression patterns of TMEM100 mRNA, we conducted RT-PCR analyses in 32 paired NSCLC tissues and normal lung tissues and obtained the consistent results. These results are in line with the research about TMEM100 in HCC [22].
Frullanti et al [14] already found that TMEM100 inhibited the clonogenicity of H460 and H520 cells, and our study confirmed the function of TMEM100 in NSCLC cells more clearly. We overexpressed exogenous TMEM100 in A549 and PC9 cells and found that TMEM100 not only suppressed cell cycle leading to A549 and PC9 cell proliferation in vitro and in vivo but inhibited cell migration and invasion. On the contrary, knocking down TMEM100 promoted NSCLC proliferation and migration, suggesting that TMEM100 may function as a tumor suppressor in NSCLC.
To further investigate which signaling pathway TMEM100 participated in and contributed to the tumor suppression function, we analyzed the gene expression profile after exogenous overexpression of TEM100 and found that most genes in the TNF pathway were downregulated, and the typical genes were verified by RT-PCR.TNF was originally discovered in the serum of animals injected sequentially with a bacterial vaccine and endotoxin. When this serum was administered intravenously, the sarcoma previously transplanted subcutaneously into the mice got necrosed [23,24]. TNF is known as a tumor suppressor, but it also contributes to cancer initiation and tumor progression by creating a permissive microenvironment for tumor invasion and metastasis [25,26]. In this study, the results of pathway GO assays demonstrated that TMEM100 was able to inhibit NSCLC cells by suppressing the TNF pathway.
In conclusion, downregulation of TMEM100 in NSCLC was associated with poor prognosis. TMEM100 possessed the potency to suppress NSCLC growth and metastasis by inhibiting the TNF pathway, and therefore may function as a tumor suppressor in NSCLC. These data may provide a wider perspective on NSCLC research and treatment.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81501927, 81201556, GJJ15026), China Postdoctoral Science Foundation (43302), and the Natural Science Foundation of Shanghai (13ZR1413900).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Patel JN, Ersek JL, Kim ES. Lung cancer biomarkers, targeted therapies and clinical assays. Transl Lung Cancer Res. 2015;4:503–514. doi: 10.3978/j.issn.2218-6751.2015.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takeda M, Okamoto I, Nakagawa K. Clinical development of nintedanib for advanced non-small-cell lung cancer. Ther Clin Risk Manag. 2015;11:1701–1706. doi: 10.2147/TCRM.S76646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Travis WD. Pathology of lung cancer. Clin Chest Med. 2002;23:65–81. doi: 10.1016/s0272-5231(03)00061-3. [DOI] [PubMed] [Google Scholar]
- 4.Goldstraw P, Ball D, Jett JR, Le Chevalier T, Lim E, Nicholson AG, Shepherd FA. Non-small cell lung cancer. Lancet. 2011;378:1727–1740. doi: 10.1016/S0140-6736(10)62101-0. [DOI] [PubMed] [Google Scholar]
- 5.Pastorino U. Lung cancer screening. Br J Cancer. 2010;102:1681–1686. doi: 10.1038/sj.bjc.6605660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Chen P, Zu L, Liu B, Wang M, Zhou Q. MicroRNA-338-3p suppresses metastasis of lung cancer cells by targeting the EMT regulator Sox4. Am J Cancer Res. 2016;6:127–140. [PMC free article] [PubMed] [Google Scholar]
- 7.Luo W, Lin Y, Meng S, Guo Y, Zhang J, Zhang W. miRNA-296-3p modulates chemosensitivity of lung cancer cells by targeting CX3CR1. Am J Transl Res. 2016;8:1848–1856. [PMC free article] [PubMed] [Google Scholar]
- 8.Tang W, Han M, Ruan B, Jin W, Lou J, Yuan X, Chen D, Chen Y, Shin VY, Jin H, Wang X. Overexpression of GOLPH3 is associated with poor survival in Non-small-cell lung cancer. Am J Transl Res. 2016;8:1756–1762. [PMC free article] [PubMed] [Google Scholar]
- 9.Liu T, Li WM, Wang WP, Sun Y, Ni YF, Xing H, Xia JH, Wang XJ, Zhang ZP, Li XF. Inhibiting CREPT reduces the proliferation and migration of non-small cell lung cancer cells by down-regulating cell cycle related protein. Am J Transl Res. 2016;8:2097–2113. [PMC free article] [PubMed] [Google Scholar]
- 10.Sang Y, Zhou F, Wang D, Bi X, Liu X, Hao Z, Li Q, Zhang W. Up-regulation of long non-coding HOTTIP functions as an oncogene by regulating HOXA13 in non-small cell lung cancer. Am J Transl Res. 2016;8:2022–2032. [PMC free article] [PubMed] [Google Scholar]
- 11.Moon EH, Kim MJ, Ko KS, Kim YS, Seo J, Oh SP, Lee YJ. Generation of mice with a conditional and reporter allele for Tmem100. Genesis. 2010;48:673–678. doi: 10.1002/dvg.20674. [DOI] [PubMed] [Google Scholar]
- 12.Kawai J, Shinagawa A, Shibata K, Yoshino M, Itoh M, Ishii Y, Arakawa T, Hara A, Fukunishi Y, Konno H, Adachi J, Fukuda S, Aizawa K, Izawa M, Nishi K, Kiyosawa H, Kondo S, Yamanaka I, Saito T, Okazaki Y, Gojobori T, Bono H, Kasukawa T. Functional annotation of a full-length mouse cDNA collection. Nature. 2001;409:685–690. doi: 10.1038/35055500. [DOI] [PubMed] [Google Scholar]
- 13.Moon EH, Kim YS, Seo J, Lee S, Lee YJ, Oh SP. Essential role for TMEM100 in vascular integrity but limited contributions to the pathogenesis of hereditary haemorrhagic telangiectasia. Cardiovasc Res. 2015;105:353–60. doi: 10.1093/cvr/cvu260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frullanti E, Colombo F, Falvella FS, Galvan A, Noci S, De Cecco L, Incarbone M, Alloisio M, Santambrogio L, Nosotti M, Tosi D, Pastorino U, Dragani TA. Association of lung adenocarcinoma clinical stage with gene expression pattern in noninvolved lung tissue. Int J Cancer. 2012;131:E643–648. doi: 10.1002/ijc.27426. [DOI] [PubMed] [Google Scholar]
- 15.Weng HJ, Patel KN, Jeske NA, Bierbower SM, Zou W, Tiwari V, Zheng Q, Tang Z, Mo GC, Wang Y, Geng Y, Zhang J, Guan Y, Akopian AN, Dong X. Tmem100 is a regulator of TRPA1-TRPV1 complex and contributes to persistent pain. Neuron. 2015;85:833–46. doi: 10.1016/j.neuron.2014.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenman ST, Gibbons SJ, Singh RD, Bernard CE, Wu J, Sarr MG, Kendrick ML, Larson DW, Dozois EJ, Shen KR, Farrugia G. Distribution of TMEM100 in the mouse and human gastrointestinal tract--a novel marker of enteric nerves. Neuroscience. 2013;240:117–128. doi: 10.1016/j.neuroscience.2013.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moon EH, Kim MJ, Ko KS, Kim YS, Seo J, Oh SP, Lee YJ. Generation of mice with a conditional and reporter allele for Tmem100. Genesis. 2010;48:673–678. doi: 10.1002/dvg.20674. [DOI] [PubMed] [Google Scholar]
- 18.Somekawa S, Imagawa K, Hayashi H, Sakabe M, Ioka T, Sato GE, Inada K, Iwamoto T, Mori T, Uemura S, Nakagawa O, Saito Y. Tmem100, an ALK1 receptor signaling-dependent gene essential for arterial endothelium differentiation and vascular morphogenesis. Proc Natl Acad Sci U S A. 2012;109:12064–12069. doi: 10.1073/pnas.1207210109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamazaki T, Muramoto M, Okitsu O, Morikawa N, Kita Y. Discovery of a novel neuroprotective compound, AS1219164, by high-throughput chemical screening of a newly identified apoptotic gene marker. Eur J Pharmacol. 2011;669:7–14. doi: 10.1016/j.ejphar.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 20.Carty CL, Johnson NA, Hutter CM, Reiner AP, Peters U, Tang H, Kooperberg C. Genome-wide association study of body height in African Americans: the Women’s Health Initiative SNP Health Association Resource (SHARe) Hum Mol Genet. 2012;21:711–720. doi: 10.1093/hmg/ddr489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Heul-Nieuwenhuijsen L, Hendriksen PJ, van der Kwast TH, Jenster G. Gene expression profiling of the human prostate zones. BJU Int. 2006;98:886–897. doi: 10.1111/j.1464-410X.2006.06427.x. [DOI] [PubMed] [Google Scholar]
- 22.Ou D, Yang H, Hua D, Xiao S, Yang L. Novel roles of TMEM100: inhibition metastasis and proliferation of hepatocellular carcinoma. Oncotarget. 2015;6:17379–17390. doi: 10.18632/oncotarget.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975;72:3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Green S, Dobrjansky A, Carswell EA, Kassel RL, Old LJ, Fiore N, Schwartz MK. Partial purification of a serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1976;73:381–385. doi: 10.1073/pnas.73.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan TT, Coussens LM. Humoral immunity, inflammation and cancer. Curr Opin Immunol. 2007;19:209–216. doi: 10.1016/j.coi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Balkwill FR, Mantovani A. Cancer-related inflammation: common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22:33–40. doi: 10.1016/j.semcancer.2011.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.