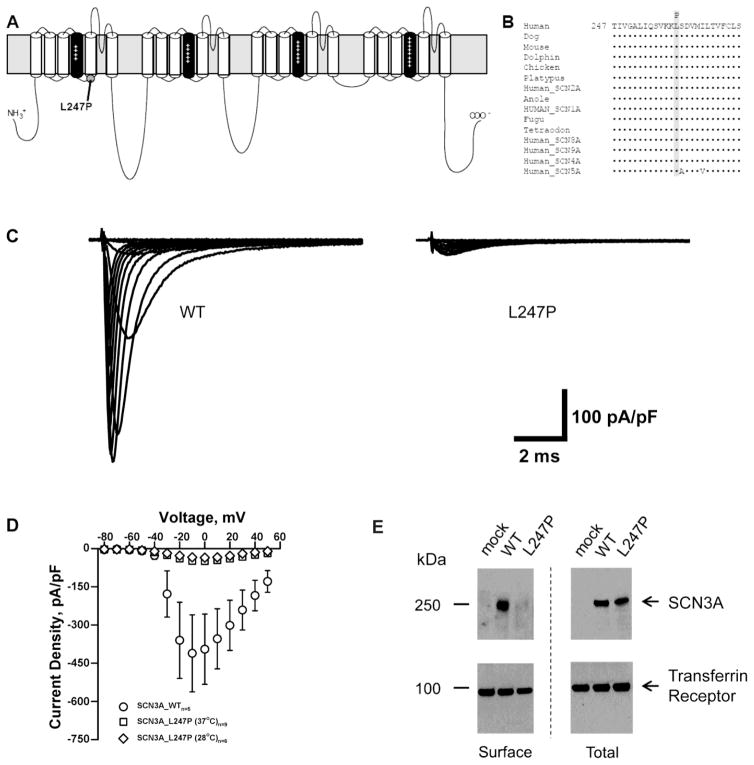
Fig. 1.
SCN3A-L247P is a trafficking deficient mutant. (A) Predicted transmembrane topology of Nav1.3 showing the location of the L247P variant characterized in this study. The S4 segments are shown in black with plus (+) signs and the grey rectangle behind the depiction represents the plasma membrane. (B) Multiple alignment of human homologs and species orthologs of Nav1.3 (Clustal Omega; (Sievers et al., 2011)). The variant amino acid L247P (shaded) is contained within a region of strong evolutionary conservation. (C) Average sodium current traces for SCN3A-WT and SCN3A-L247P co-expressed with β1 and β2 in a heterologous expression system (tsA201 cells). (D) Average current density-voltage relationships are shown for whole-cell currents tsA201 cells transiently co-expressing β1 and β2 with SCN3A-WT or SCN3A-L247P incubated at 37°C or 28°C for 24 hours prior to recording. (E) Total and surface protein were detected with anti-pan VGSC α subunit antibody or anti-transferrin receptor antibody as indicated. The WT SCN3A protein traffics normally to the cell surface, whereas trafficking of SCN3A L247P to the cell surface is significantly impaired.