Figure 1.
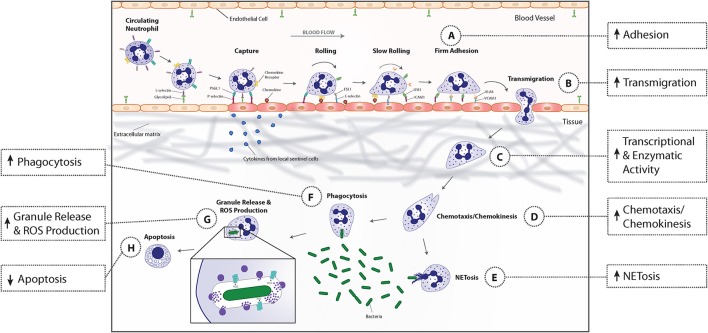
Priming-associated phenotypic changes and their effect on neutrophil functional responses. Neutrophils in circulating blood are in a resting state, characterized by a round morphology, non-adherence, minimal transcriptional activity, and a limited capacity to respond to activating stimuli. Microbial entry into tissues or tissue injury induces local immune cells to release pro-inflammatory cytokines that modify endothelial cell adhesion molecule profile and enter the bloodstream to begin priming neutrophils. Upon exposure to these priming agents, neutrophils undergo an increase in enzymatic and transcriptional activity that results in activation and synthesis of inflammatory mediators and enzymes that mediate downstream phenotypic and functional changes. Immediately, neutrophils begin to change their adhesion receptor pattern by shedding selectins, fusing secretory vesicles with the plasma membrane which leads to increased integrin expression, and a rapid increase in the gene expression of several surface receptors that allows newly primed cells to more rapidly adhere to endothelial cells (A). This phenotypic change coupled with the release of granules containing matrix metalloproteases, promotes neutrophil migration into inflamed tissues (B). The priming process continues when neutrophils bind to extracellular matrix proteins (C). Binding of neutrophil extracellular matrix receptors leads to an increase in actin polymerization, available receptors from secretory vesicle degranulation, and intracellular signaling that results in enhanced chemotaxis and chemokinesis (D). When primed neutrophils encounter bacteria, their phagocytic capacity is increased due to the upregulation in the number and affinity of receptors on the plasma membrane (F). By then, ROS production, granule release (G), and NET formation (E) have been primed to augment microbicidal activities. Finally, priming prolongs neutrophil lifespan by activating anti-apoptotic signal transduction pathways and transcription factors that decrease transcription of pro-apoptotic factors (H).