Abstract
Aims: Sarcodon imbricatus, an edible fungus, is widely used in Asian medicine because of its significant pharmacological activities. In the present study, we investigated the immunomodulatory effects of polysaccharide-enriched S. imbricatus extracts (SP) in cyclophosphamide (CTX)-induced immunosuppressed mice. Results: Astragalus polysaccharide (AP) was used as a positive control. Compared with CTX-induced immunosuppressed mice, thirty-day SP treatment strongly enhanced the organ indexes of spleen and thymus and suppressed hind paw swelling. Both AP and SP increased the serum levels of immunoglobulin (IgA, IgG, and IgM), and suppressed the overproduction of interleukin-2 (IL-2). Moreover, SP reduced methane dicarboxylic aldehyde levels, and increased the total antioxidant capacity, superoxide dismutase, and glutathione peroxidase in both serum and liver tissues of CTX-induced immunosuppressed mice. Conclusion: S. imbricatus extracts significantly improved immune function in CTX-induced immunosuppressed mice via modulation of oxidative systems.
Keywords: Sarcodon imbricatus, Immunity, Cyclophosphamide, Oxidation
1. Introduction
Immunity is a physiological function of human bodies that identifies “self” and “non-self” components, undermines and excludes antigenic material, and maintains human health (Viladomiu et al., 2016, Halim et al., 2017). Immunological homeostasis, immune surveillance, and immune defense are three immune system functions. Immunosuppression is observed in various pathophysiological and clinical conditions (Wang et al., 2015, Chouhan et al., 2015). Cyclophosphamide (CTX) is clinically used for tumor therapy and exhibits strong immunosuppressive effects in patients, by causing damage to the immune system and hematopoietic function (Safi et al., 2015a, Safi et al., 2015b, Diaz-Montero et al., 2012). In addition, CTX causes senescence by disturbing the balance of the oxidation system.
Commonly used regulatory medicines may exhibit adverse effects, including general malaise and/or neurotoxicity (Wang et al., 2015, Luo et al., 2016, Plonka, 2015, Markman, 1996). Numerous studies have demonstrated that active ingredients derived from herbs, such as polysaccharides, alkaloids, and saponins, can modulate immune function in humans and animals (Kuang et al., 2011). By regulating the oxidation system and relieving inflammation, Cordyceps militaris fruit bodies improved membranous glomerulonephritis in rats (Song et al., 2016, Yine et al., 2015a, Yine et al., 2015b, Muhammad et al., 2017). Previously, we found that Tricholoma matsutake enhanced immunity in CTX-induced immunosuppressed mice by promoting the secretion of immunomodulatory molecules. Sarcodon imbricatus, a well-known edible fungus, has been widely used in Asian medicine because of its pharmacological activities (Khaliq et al., 2016, Vizzini et al., 2012). However, most studies regarding S. imbricatus have focused on the separation and analysis of its components (Sułkowska-Ziaja et al., 2014, Ishaq and Jafri, 2017); its immunomodulatory function has not previously been systematically studied.
In the present study, the immunomodulatory activities of S. imbricatus were determined in CTX-induced immunosuppressed mice. The mechanisms of S. imbricatus-mediated immunomodulatory effects were determined by analyzing the levels of immunoglobulin, inflammatory factors, and oxidation related kinases.
2. Methodology
2.1. Animal experiments
S. imbricatus fruit bodies were extracted twice with 20-fold double distilled (D.D.) water at 95 °C for 4 h. The supernatant was collected and freeze dried prior to use. Polysaccharide-enriched S. imbricatus extracts were denoted as SP.
The experimental protocol was approved by the Institution Animal Ethics Committee of Jilin University. Six-week-old KunMing (KM) mice (18–22 g) were purchased from the Experimental Research Center of Medical Animal (Guangdong, China, SCXK(YUE)2013-0002), and housed under a 12-h light/dark cycle at 23 ± 1 °C with water and food available ad libitum.
Sixty mice were injected with CTX (40 mg/kg) subcutaneously for seven days. An additional 12 mice were injected with normal saline and used as a control group (CTRL). CTX-treated immunosuppressed mice were randomly separated into five groups (n = 12), and orally treated with either 80 mg/kg of Astragalus polysaccharide (AP + CTX group; positive control mice) or 50, 100, or 200 mg/kg of SP (SP + CTX groups) for thirty days. Control mice were orally treated with 0.1 mL/kg of normal saline for thirty days. The body weight of each mouse was measured on days 1, 10, and 30.
After delayed-type hypersensitivity testing, blood was sampled from each group. The mice were then sacrificed via injection with 200 mg/kg of pentobarbital. The liver, spleen, and thymus of each mouse were removed and weighed. Organ indexes were calculated as the organ weight divided by the total body weight.
2.2. Delayed-type hypersensitivity (DTH) tests
On day 26, the mice were sensitized via intraperitoneal injection with 0.2 mL of 2% (v/v) sheep red blood cells (SRBC) for four days. The sensitized mice were challenged via injection with 20 μl of 20% (v/v) SRBC into the measured hind paw. The thickness of the hind paw was recorded at 24 h post-injection.
2.3. Biochemical index measurements
The levels of oxidation factors (methane dicarboxylic aldehyde (MDA), total antioxidant capacity (T-AOC), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px)), immunoglobulin (IgA, IgG, and IgM), and interleukin-2 (IL-2) in serum and/or liver tissues were determined using enzyme-linked immunosorbent assay (ELISA) kits (Calbiotech, USA) following the manufacturer’s protocols.
2.4. Statistical analyses
Data were expressed as mean ± S.D. A one-way analysis of variance (ANOVA) followed by Dunn’s test using SPSS 17.0 software (IBM Corporation, Armonk, USA) were used to determine statistical significance. P < 0.05 was recognized as significant.
3. Results and discussion
3.1. Effects of SP on organ indexes and hind paw swelling
Low thymus and spleen indexes were exhibited by CTX-induced immunosuppressed mice (P < 0.05; Table 1). In contrast, SP at 100 and 200 mg/kg increased spleen indexes, and AP at 80 mg/kg and SP at all of the administered doses improved thymus indexes (P < 0.05; Table 1). The thymus transports activated T cells into blood circulation and promotes mast cell development (Li et al., 2015, Rahman et al., 2017). The spleen synthesizes immune effector molecules and promotes phagocytosis of granulocytes (Hassanpour et al., 2013, Atta et al., 2017). Hence, the enhanced organ indexes exhibited by SP-treated mice are beneficial to immunoregulatory functions.
Table 1.
Effects of SP and AP on hind paw swelling and organ indexes of spleen and thymus.
| Spleen index | Thymus index | Hand paw swelling | ||
|---|---|---|---|---|
| CTRL | 2.0 ± 0.2 | 1.8 ± 0.1 | 0.9 ± 0.3 | |
| Model | 1.6 ± 0.2## | 1.1 ± 0.4## | 0.3 ± 0.2### | |
| SP (mg/kg) | 50 | 1.8 ± 0.4 | 1.6 ± 0.4* | 0.3 ± 0.1 |
| 100 | 1.9 ± 0.4* | 1.7 ± 0.3* | 0.4 ± 0.1 | |
| 200 | 2.1 ± 0.5* | 1.8 ± 0.4** | 0.6 ± 0.3* | |
| AP (mg/kg) | 80 | 1.9 ± 0.3 | 1.8 ± 0.7* | 0.8 ± 0.3** |
Data are expressed as mean ± S.D. (n = 12). CTX: cyclophosphamide. SP: S. imbricatus extracts. AP: Astragalus polysaccharides.
P < 0.05 versus control group.
P < 0.001 versus control group.
P < 0.05 versus CTX mice.
P < 0.01 versus CTX mice.
SP and AP significantly suppressed hind paw swelling compared to CTX-induced immunosuppressed mice (P < 0.05; Table 1). Hind paw swelling indicates the strength of delayed allergy (DTH), which is mediated by T cells involved in cellular immunity (Huang et al., 2015, Dai et al., 2014, Sarfraz et al., 2017). Data suggest that the immunomodulatory activity of SP may be related to cellular immunity.
3.2. Upregulation of immunoglobulin and IL-2 by SP
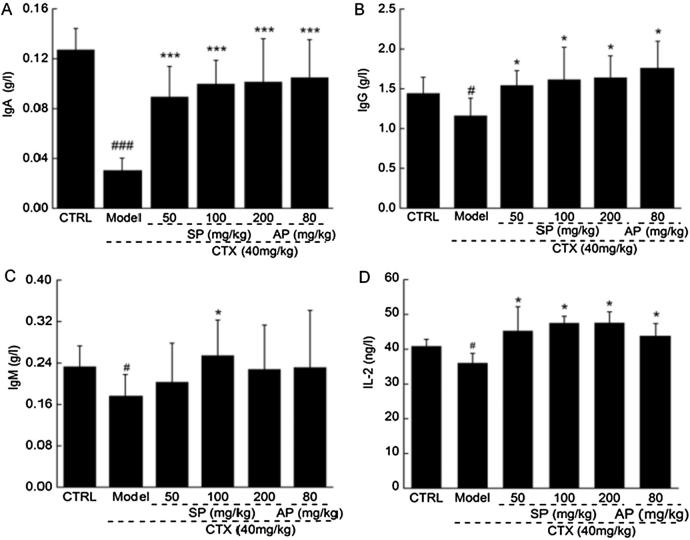
Low immunoglobulin levels (including IgA, IgG, and IgM) were found in CTX-induced immunosuppressed mice. Immunoglobulin levels were significantly increased after thirty-day SP treatment (P < 0.05; Fig. 1A–C). Foreign substances can be identified and neutralized by immunoglobulin. During humoral immune response, opsonophagocytosis is initiated by increased IgA and IgG levels upon antigen exposure. Thus, humoral immunity may be involved in SP-mediated immunomodulatory effects in CTX-induced immunosuppressed mice.
Fig. 1.
SP-regulated serum levels of (A) IgA, (B) IgG, (C) IgM, and (D) IL-2 in CTX-induced immunosuppressed mice. Data are expressed as mean ± S.D. (n = 12). #P < 0.05 and ###P < 0.001 versus control group. *P < 0.05 and ***P < 0.001 versus CTX mice. CTX: cyclophosphamide. SP: S. imbricatus extracts. AP: Astragalus polysaccharides.
Lower levels of IL-2 were determined in CTX mice than in non-treated mice (P < 0.05; Fig. 1D). Thirty-day SP treatment resulted in >25% enhancement of IL-2 levels (P < 0.05; Fig. 1D). Cytokines are necessary for the differentiation of memory T cells. Perturbations in cytokines levels may cause various pathologies, including immunodeficiency, autoimmunity, and atopic disease (Sun et al., 2011). IL-2, a pleiotropic cytokine, drives T-cell growth, augments NK cytolytic activity, induces the differentiation of regulatory T cells, and mediates activation-induced cell death (Zhao and Ashraf, 2016, Liao et al., 2011). Therefore, the immune regulatory activities of SP may be partially attributed to the regulation of immunoglobulin and IL-2 levels.
3.3. SP regulation of oxidative factors
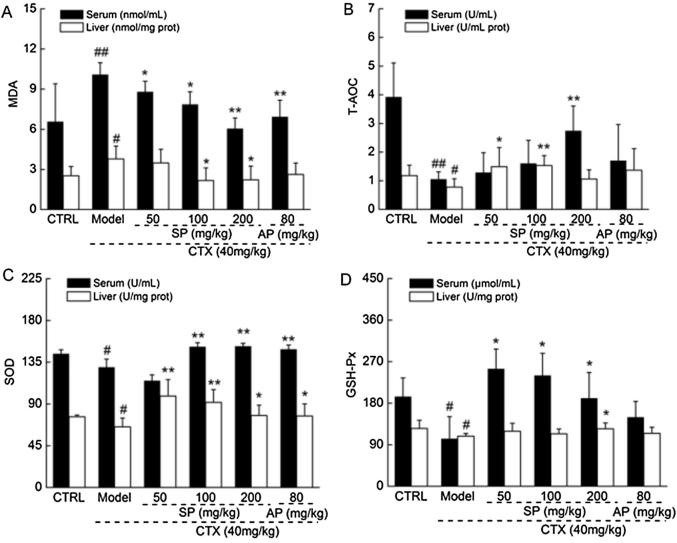
MDA levels directly indicate the degree of lipid oxidation in the body and indirectly indicate the degree of cell damage. SOD, GSH-Px, and AOC levels represent the ability of an organism to remove oxygen free radicals. Compared to control mice, CTX enhanced MDA levels, and significantly reduced the activities of SOD, GSH-Px, and AOC in serum and liver tissues (P < 0.05; Fig. 2). These levels were normalized by SP treatment (P < 0.05; Fig. 2).
Fig. 2.
SP-regulated levels of (A) MDA, (B) T-AOC, (C) SOD, and (D) GSH-Px in serum and liver tissues of CTX-induced immunosuppressed mice. Data are expressed as mean ± S.D. (n = 12). #P < 0.05 and ##P < 0.01 versus control group. *P < 0.05 and **P < 0.01 versus CTX mice. CTX: cyclophosphamide. SP: S. imbricatus extracts. AP: Astragalus polysaccharides.
Reactive oxygen species (ROS) are highly reactive molecules containing oxygen, and can modify DNA and proteins (Zafar et al., 2016). Under normal conditions, ROS are balanced by the antioxidation defense system, including SOD and GSH-Px. When an imbalance between oxidants and antioxidants occurs, oxidative stress is reached (Portal-Nunez et al., 2016). SOD and GSH-Px protect against oxidative cell damage by clearing excessive MDA and ROS (Ma et al., 2016, Sies, 1997). Previously, we demonstrated that Cordyceps militaris protects rats against membranous glomerulonephritis by relieving oxidative damage (Liu et al., 2015, Sarfraz et al., 2016, Song et al., 2016). Overall, SP-mediated immunomodulatory activity may be attributed to its modulation of the oxidation system.
4. Conclusion
S. imbricatus extracts enhanced the immunity of CTX-induced immunosuppressed mice by increasing immunoglobulin and immune factors in serum, enhancing organ indexes of thymus and spleen, and neutralizing oxidative stress. Further investigation is necessary to determine the applicability of SP to immune-related diseases.
Acknowledgements
This work was supported by Science and Technology Key Project in Jilin Province of China (Grant No. 20140311072YY, 20150203002NY and 20160204029YY).
Footnotes
Peer review under responsibility of King Saud University.
References
- Atta A., Mustafac G., Sheikh M.A., Shahid M., Xiao H. The biochemical significances of the proximate, mineral and phytochemical composition of selected vegetables from Pakistan. Matrix Sci. Pharma. 2017;1(1):06–09. [Google Scholar]
- Chouhan G., Islamuddin M., Want M.Y., Abdin M.Z., Ozbak H.A., Hemeg H.A. Apoptosis mediated leishmanicidal activity of Azadirachta indica bioactive fractions is accompanied by Th1 immunostimulatory potential and therapeutic cure in vivo. Parasites Vectors. 2015;8:183. doi: 10.1186/s13071-015-0788-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai M.M., Wu H., Li H., Chen J., Chen J.Y., Hu S.L. Effects and mechanisms of Geniposide on rats with adjuvant arthritis. Int. Immunopharmacol. 2014;20(1):46–53. doi: 10.1016/j.intimp.2014.02.021. [DOI] [PubMed] [Google Scholar]
- Diaz-Montero C.M., Wang Y., Shao L., Feng W., Zidan A.A., Pazoles C.J. The glutathione disulfide mimetic NOV-002 inhibits cyclophosphamide-induced hematopoietic and immune suppression by reducing oxidative stress. Free Radical Biol. Med. 2012;52(9):1560–1568. doi: 10.1016/j.freeradbiomed.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halim A.N.I., Huyap F., Hamid A.T.T.H., Halim A.K.B., Hamid A.A.A. In silico binding interactions of dehalogenase (Dehe) with various haloalkanoic acids. Galeri Warisan Sains. 2017;1(1):04–06. [Google Scholar]
- Hassanpour M., Joss J., Mohammad M.G. Functional analyses of lymphocytes and granulocytes isolated from the thymus, spiral valve intestine, spleen, and kidney of juvenile Australian lungfish, Neoceratodus forsteri. Fish Shellfish Immunol. 2013;35(1):107–114. doi: 10.1016/j.fsi.2013.04.006. [DOI] [PubMed] [Google Scholar]
- Huang X., Deng L., Lu G., He C., Wu P., Xie Z., Ashraf M.A. Research on the treatment of Pseudomonas aeruginosa pneumonia in children by macrolide antibiotics. Open Med. 2015;2015(10):479–482. doi: 10.1515/med-2015-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishaq S., Jafri S. Biomedical importance of cocoa (Theobroma cacao): Significance and potential for the maintenance of human health. Matrix Sci. Pharma. 2017;1(1):01–05. [Google Scholar]
- Khaliq T., Sarfraz M., Ashraf M.A. Recent progress for the utilization of curcuma longa, Piper nigrum and Phoenix dactylifera seeds against type 2 diabetes. West Indian Med. J. 2016;64(5):527–532. doi: 10.7727/wimj.2016.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang H., Xia Y., Yang B., Wang Q., Wang Y. Screening and comparison of the immunosuppressive activities of polysaccharides from the stems of Ephedra sinica Stapf. Carbohyd. Polym. 2011;83(2):787–795. [Google Scholar]
- Liao W., Lin J.X., Leonard W.J. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr. Opin. Immunol. 2011;23(5):598–604. doi: 10.1016/j.coi.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Ji P.Y., Song L.G., Lei J.X., Lv Z.Y., Wu Z.D. The expression of molecule CD28 and CD38 on CD4(+)/CD8(+) T lymphocytes in thymus and spleen elicited by Schistosoma japonicum infection in mice model. Parasitol. Res. 2015;114(8):3047–3058. doi: 10.1007/s00436-015-4507-y. [DOI] [PubMed] [Google Scholar]
- Liu P., Meng W., Wang S., Sun Y., Ashraf M.A. Quaternary ammonium salt of chitosan: preparation and antimicrobial property for paper. Open Med. 2015;2015(10):473–478. doi: 10.1515/med-2015-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Wang X., Yang Y., Lan T., Ashraf M.A., Mao Q. Successful treatment of cerebral aspergillosis in a patient with acquired immune deficiency syndrome. West Indian Med. J. 2016;64(5):540–542. doi: 10.7727/wimj.2016.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J.Q., Luo R.Z., Jiang H.X., Liu C.M. Quercitrin offers protection against brain injury in mice by inhibiting oxidative stress and inflammation. Food Funct. 2016;7(1):549–556. doi: 10.1039/c5fo00913h. [DOI] [PubMed] [Google Scholar]
- Markman M. Chemotherapy-associated neurotoxicity: an important side effect-impacting on quality, rather than quantity, of life. J. Cancer Res. Clin. Oncol. 1996;122(9):511–512. doi: 10.1007/BF01213547. [DOI] [PubMed] [Google Scholar]
- Muhammad G., Rashid I., Firyal S., Saqib M. Successful treatment of idiopathic generalized subcutaneous emphysema in kajli a ram by large bore injection needle. Matrix Sci. Medica. 2017;1(1):01–02. [Google Scholar]
- Plonka P.M. Hair pigmentation disorders or 50 years of German-Polish alliance for study on a severe side effect of chemotherapy: Kostanecki's legacy. Exp. Dermatol. 2015;24(1):10–11. doi: 10.1111/exd.12560. [DOI] [PubMed] [Google Scholar]
- Portal-Nunez S., Esbrit P., Alcaraz M.J., Largo R. Oxidative stress, autophagy, epigenetic changes and regulation by miRNAs as potential therapeutic targets in osteoarthritis. Biochem. Pharmacol. 2016;108:1–10. doi: 10.1016/j.bcp.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Rahman A.S., Kahar A.A., Mansor A., Murni D.L., Hussin A., Sharifudin A.S., Hun T.G., Rashid A.N.Y., Othaman M.A., Long K. Identification of potential indigenous microbe from local fermented vegetables with antimicrobial activity. Galeri Warisan Sains. 2017;1(1):01–03. [Google Scholar]
- Safi S.Z., Batumalaie K., Mansor M., Chinna K., Mohan S., Karimian H., Qvist R., Ashraf M.A., Yan G.O. Glutamine treatment attenuates hyperglycemia-induced mitochondrial stress and apoptosis in umbilical vein endothelial cells. Clinics. 2015;70(8):569–576. doi: 10.6061/clinics/2015(08)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safi S.Z., Qvist R., Chinna K., Ashraf M.A., Paramasivam D., Ismail I.S. Gene expression profiling of the peripheral blood mononuclear cells of offspring of one type 2 diabetic parent. Int. J. Diabetes Dev. Countries. 2015;2015:1–8. [Google Scholar]
- Sarfraz M., Ashraf Y., Sajid S., Ashraf M.A. Testosterone level in testicular cancer patients after chemotherapy. West Indian Med. J. 2016;64(5):487–494. doi: 10.7727/wimj.2016.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarfraz M., Ashraf Y., Ashraf S. A review: prevalence and antimicrobial susceptibility profile of listeria species in milk products. Matrix Sci. Medica. 2017;1(1):03–09. [Google Scholar]
- Sies H. Oxidative stress: oxidants and antioxidants. Exp. Physiol. 1997;82(2):291–295. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- Song J., Wang Y., Liu C., Huang Y., He L., Cai X. Cordyceps militaris fruit body extract ameliorates membranous glomerulonephritis by attenuating oxidative stress and renal inflammation via the NF-kappaB pathway. Food Funct. 2016;7(4):2006–2015. doi: 10.1039/c5fo01017a. [DOI] [PubMed] [Google Scholar]
- Sułkowska-Ziaja K., Muszyńska B., Ekiert H. Analysis of indole compounds from the fruiting bodies and the culture mycelia of Sarcodon imbricatus. Mycoscience. 2014;55(3):164–167. [Google Scholar]
- Sun Y., Asmal M., Lane S., Permar S.R., Schmidt S.D., Mascola J.R. Antibody-dependent cell-mediated cytotoxicity in simian immunodeficiency virus-infected rhesus monkeys. J. Virol. 2011;85(14):6906–6912. doi: 10.1128/JVI.00326-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viladomiu M., Hontecillas R., Bassaganya-Riera J. Modulation of inflammation and immunity by dietary conjugated linoleic acid. Eur. J. Pharmacol. 2016;785:87–95. doi: 10.1016/j.ejphar.2015.03.095. [DOI] [PubMed] [Google Scholar]
- Vizzini A., Carbone M., Boccardo F., Ercole E. Molecular validation of Sarcodon quercinofibulatus, a species of the S. imbricatus complex associated with Fagaceae, and notes on Sarcodon. Mycol. Prog. 2012;12(3):465–474. [Google Scholar]
- Wang D., Fu Y.B., Ashraf M.A. Artifacts reduction in strain maps of tagged magnetic resonance imaging using harmonic phase. Open Med. 2015;10:425–433. doi: 10.1515/med-2015-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yine H., Shufang D., Bin W., Wei Q., Ashraf M.A., Junling G. Application of food-specific IgG antibody detection in allergy dermatosis. Open Med. 2015;2015(10):377–381. doi: 10.1515/med-2015-0067. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yine H., Shufang D., Bin W., Wei Q., Junling G., Ashraf M.A. Analysis of the relations between allergen specific LgG antibody and allergic dermatosis of 14 kinds foods. Open Med. 2015;10:405–409. doi: 10.1515/med-2015-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar A., Singh S., Naseem I. Cu(II)-coumestrol interaction leads to ROS-mediated DNA damage and cell death: a putative mechanism for anticancer activity. J. Nutr. Biochem. 2016;33:15–27. doi: 10.1016/j.jnutbio.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Zhao L., Ashraf M.A. Influence of Ag/HA nanocomposite coating on biofilm formation of joint prosthesis and its mechanism. West Indian Med. J. 2016;64(5):506–513. doi: 10.7727/wimj.2016.179. [DOI] [PMC free article] [PubMed] [Google Scholar]