Abstract
We report a case of a laboratory-confirmed Dengue and Chikungunya viruses co-infection imported from India to Portugal in early November 2016. The patient developed fever, retro-orbital pain and generalized myalgia after returning from Delhi, Jaipur, Agra, Rishikesh, Goa and Mumbai. This case highlights the importance of these arboviruses to public health in India where high rates of co-infection have been reported in the last few years, and demonstrates how challenging the laboratory diagnosis of imported co-infection cases can be in non-endemic areas.
Keywords: Dengue virus, Chikungunya virus, Co-infection, Arboviruses
Introduction
Dengue and Chikungunya are two mosquito-borne viral diseases transmitted by Aedes species, mainly Ae. aegypti, that, together with Zika virus, are nowadays co-circulating in a wide geographic area, covering African, Asian and Latin and South American regions. The infections of these three single-stranded positive-sense RNA genome arboviruses share similar primary signs and symptoms and represent a significant burden for the healthcare systems.
Dengue virus (DENV) (family Flaviviridae, genus Flavivirus) is the etiological agent of dengue fever, an infection that cause a wide spectrum of human disease, from asymptomatic cases to classic dengue fever and more severe cases, that is endemic in the tropics and subtropics. Four distinct serotypes (DENV-1, DENV-2, DENV-3 and DENV-4) that share all the above clinical manifestations are recognized. Infection with one of the four serotypes of DENV confers lifelong immunity to that specific serotype, but is only partial and temporary to the remaining serotypes. Therefore, an individual can have at the most four DENV infections and the risk of severe infections is increased in subsequent infections by other serotypes [1]. The incidence of dengue has grown widely in the last decades, being actual numbers of dengue cases underreported and in many cases are misclassified [2]. Estimates indicate that about 390 million DENV infections occur annually [3] and 3.9 billion people in 128 countries are at risk of infection with dengue viruses [4]. Case fatality rates vary between 0.5%–3.5% [5]. Symptoms usually last for 2–7 days, after an incubation period of 4–10 days after the bite from an infected mosquito.
Chikungunya virus (CHIKV) is an alphavirus (family Togaviridae) firstly detected in 1952 in Makonde, United Republic of Tanzania. The virus name derives from the Swahili word kungunyala that refers the contorted posture of patients due to the painful polyarthralgia symptoms. Traditionally, CHIKV was not considered a life-threatening infection but recent epidemiological evidence indicates a case fatality rate around 0.1% [6]. There are three divergent evolutionary clades: West African, Central/East African and Asian CHIKV [7]. Since 2003, there has been a resurgence of CHIKV outbreaks that spread globally though international trade and travel, leading to autochthonous transmission events in the islands of the Pacific Ocean, in Reunion Island in 2006 and followed by India [6], [8]. Almost 1.3 million suspected CHIKV fever cases were reported in India [9]. In 2007 an outbreak of CHIKV in Italy was associated with the mutation A226 V in the E1 gene causing significant increase in vector capacity for transmission by Aedes albopictus mosquitoes [6]. Symptoms generally start 4–7 days after the mosquito bite. The acute phase is characterized by severe arthralgia, high fever, asthenia, headache, vomiting, rash and myalgia. After this initial stage, some patients experience relapse or persistent symptoms, being arthralgia or musculoskeletal pains the more frequently reported. In the outbreak on Reunion island, 53% of the patients still reported symptoms 128 days after the disease onset, and after the 2006 epidemic in West India, 12% and 5% suffered from musculoskeletal pains and arthritis at one and two years after the disease onset, respectively [10]. At least half of patients with CHIKV infection may develop chronic rheumatologic sequelae [11], namely patients older than 40 years of age, which might be misdiagnosed as an auto-immune disorder per se, even in patients with past history of DENV, if co-infection at the time is not thought of.
Case report
A 65 year old Portuguese woman, with a past medical history of sub-clinical hyperthyroidism, developed on November 8th 2016, two days after a two week trip to India (passing through New Dehli, Jaipur, Agra, Rishikesh, Goa and Mumbai), fever (38.5 °C), myalgia, retro-orbital pain, pruritus and subtle rash under tanned skin. The patient denied nausea, vomiting or any abdominal distress. During her trip, on the 31st of October she recounts having had 3 days of high grade fever (39–40 °C), intense myalgia and retro-orbital pain and was diagnosed with urinary tract infection at an Indian clinic. Tests performed showed “leukocytes and bacteria in the urine” and negative results for NS1 antigen, IgM and IgG for Dengue virus and Plasmodium spp. immunochromatographic test. She was treated with antimicrobials and was apyretic after four days.
In Portugal, at admission at the hospital, on the second day post onset of symptoms, the patient was hemodynamically stable, acyanotic, anicteric, febrile (38.6 °C), presenting signs of dehydration, discrete conjunctival hyperemia, oropharynx hyperemia with tonsillar enlargement, complaining of asthenia and anorexia. Blood tests showed lymphopenia (700 cells/μL; reference 1000–4800 cells/μL), no other hematological dysfunction, no elevation of C-reactive protein (CRP) and normal liver enzymes. After 24 h upon observation discrete hypotension, pulmonary auscultation with bilateral basal crackles and saturation of 92%, discrete peripheral edema and slowed psychomotor functioning and drowsiness, was noted, as well as new-onset of leukopenia (2580/μL; reference 4000–11,000 cells/μL) and anemia (13.3 » 11.4 g/dL; reference 12–16 g/dL). Three blood smears were negative for Plasmodium spp. Serological tests for HIV1 and 2, HBV, HCV, HAV IgM and VDRL/TPHA were negative. Echocardiography and chest X-ray did not reveal significant alterations.
After four days of hospitalization the fever and pruritus resolved (six days after onset) and she maintained diminished urinary output. Clinical improvement was observed the following days, and mild arthralgias of the wrists, elbows and shoulders began on the 5th day in the ward. Laboratory findings showed maintenance of low values for CRP, negative procalcitonin (PCT), new onset of thrombocytopenia with 56,000 platelets/μL (reference 150,000–400,000/μL) and aggravated cytocholestasis with elevated aspartate aminotransferase (AST/GOT) 295 U/L (reference <34 U/L), alanine aminotransferase (ALT/GPT) 203 U/L (reference <55 U/L) and γ-glutamyl transferase (GGT) 81 U/L (reference interval 12–64 U/L). Alkaline phosphatase (ALP) 97 U/L (reference interval of 40–150) and total bilirubin were both normal. The patient was discharged after six days (8th after onset) with improved clinical condition and resolution of analytical dysfunctions.
In a follow up clinical consultation, 15 days after the onset of symptoms, the patient presented severe polyarthralgia with predominance in the large and axial joints, showing severe difficulties in upright posture maintenance and in gait. The patient initiated symptomatic treatment with nonsteroidal anti-inflammatory drugs (NSAID) for three additional weeks with moderate improvement in arthralgias complaints. The moderate inflammatory arthralgia persisted, specially located in the hands, shoulders and tibiotarsal joint, and some restrains to arm and leg movements could still be observed. The patient developed NSAID intolerance, so a low dose of corticosteroids was started, with symptoms resolution in two weeks.
The molecular and serologic tests were performed at the National Reference Laboratory for vector borne diseases, National Institute of Health. Nucleic acids were extracted from 400 μL of ethylenediaminetetraacetic acid (EDTA) blood samples using NucliSens easyMAG platform (BioMérieux). The presence of DENV and CHIKV RNA was checked by real-time PCR [12] and by conventional RT-PCR [13], [14], respectively. Trioplex Real-time RT-PCR Assay–CDC, for DENV, CHIKV and ZIKV simultaneous detection was also tested. For DENV sequence analysis RT-PCR targeting the core pre-membrane (CprM) region [15], [16] was performed and the nested-PCR fragment was sequenced bi-directionally and submitted to GenBank (accession number KY412881). Similarity searches were made within the GenBank data set using the basic local alignment search tool (BLAST) BLASTN algorithm [17]. DENV-3 sequences used in previous studies [18], [19] and other accessible from the National Center for Biotechnology Information (NCBI) GenBank database (http://www.ncbi.nlm.nih.gov) were used as dataset. The sequences were first aligned by Clustal W and the best fit model for nucleotide substitution was identified using Mega version 6 software [20]. Maximum likelihood analysis was performed with Kimura two-parameter model with Gamma distributed (G) rate among sites (the determined best fit model) using Mega 6. The robustness of the nodes was tested by 1000 bootstrap replications.
Sera samples were tested by immunofluorescent assay (IFA) in-house (IgG and IgM) for Dengue (DENV) and Chikungunya viruses (CHIKV) (Table 1).
Table 1.
Molecular and serologic diagnostic results for DENV and CHIKV.
| Sample | 1st | 2nd | 3rd | 4th | |
|---|---|---|---|---|---|
| Days post DENV symptoms onset | 2 | 6 | 15 | 42 | |
| Dengue virus | RT-PCR Blood | +(Ct = 19.17/23.44) | NT | NT | NT |
| IgMa | – | – | +64 | – | |
| IgGb | – | – | +512 | +256 | |
| Chikungunya virus | RT-PCR Blood | – | NT | NT | NT |
| IgMa | +64 | +256 | +256 | – | |
| IgGb | +128 | +512 | +1024 | +256 | |
(+) positive; (−) negative; Ct: Cycles threshold; NT: not tested.
IgM cut-off value 16.
IgG cut-off value 32.
DENV RT-PCR was positive by real time RT-PCR (Ct = 23.44, [12]; Ct = 19.17, Trioplex Real-time RT-PCR Assay, CDC) on a blood sample collected on day 2 post onset of disease (Table 1). The same sample was negative for the presence of CHIKV and ZIKV RNA.
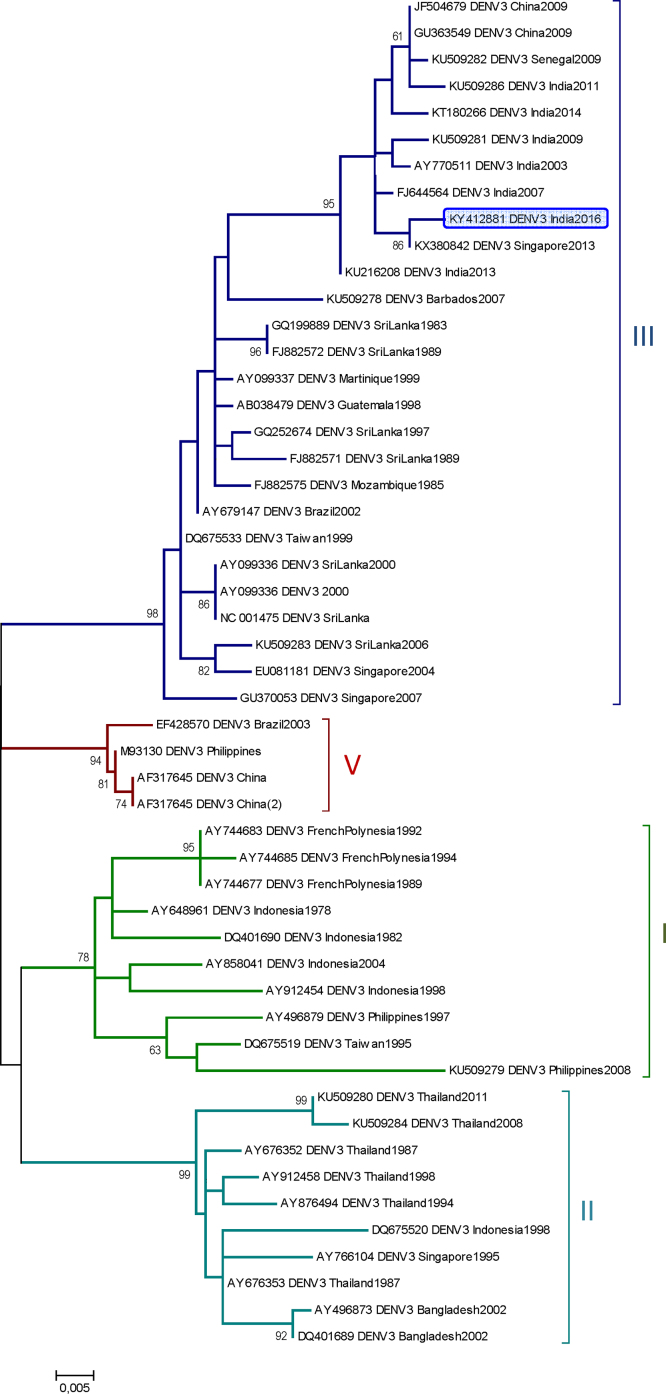
Dengue virus serotype 3 genotype III was determined by C-PrM phylogenetic analysis (Fig. 1). This was the first analyzed PCR positive sample in the laboratory for serotype 3, ruling out any possibility of PCR contamination. Besides the molecular diagnosis, the serological results showed IgM (64) and IgG (512) detection in the 3rd sample (15 days after symptoms onset) and only IgG (256) in the 4th sample (42 days after symptoms onset) (Table 1).
Fig. 1.
Phylogenetic analysis of DENV-3 sequences using partial C-PrM sequence region.
Maximum-likelihood phylogenetic tree was inferred based on 49 DENV 3 partial Capsid-Pre membrane region (C-prM) nucleotide sequences (459 bp) by using Molecular Evolutionary Genetics Analysis (MEGA) version 6 software. Distance matrices were calculated using Kimura two-parameter model with Gamma distributed (G) rate among sites. Bootstrap values obtained from 1000 replicate trees are shown for key nodes (more than 70%). Scale is shown at the bottom as substitutions per site. GenBank accession number, place and year of isolation are indicated. Dengue 3 sequence related to this work is highlighted in blue. Cluster genotypes are identified in different colors and by the respective number in the left.
CHIKV infection, although negative by molecular assays, was confirmed by the compatible clinical criteria and the presence of virus specific IgM antibodies in three successive serum samples and a four-fold rising of IgG titers in samples collected two weeks apart (Table 1).
Discussion
CHIKV and DENV usually cause a self-limited, febrile illness that is generally associated with arthralgias and myalgias. Albeit both infections can present biphasic fever [21], our results regarding the detection of DENV3 RNA and negative IgM and IgG for DENV in the first samples, and CHIKV diagnostics results, indicate that the two fever peaks in our patient (the first diagnosed as a urinary infection in India and the second after returning to Portugal), may indicate a sequential infection by the two arboviruses (first CHIKV shortly followed by DENV).
A recent study [22] highlights the likelihood of misdiagnosis of CHIKV infections in DENV endemic territories (and vice versa) and the clinical importance and outcome for infected patients. For instance, the inappropriate prescription of arthralgia-alleviating NSAIDs (especially acetylsalicylic acid) in CHIKV misdiagnosed infections (sometimes also because of misdiagnosis with other rheumatic disorders) can lead to severe bleeding in patients with dengue fever with thrombocytopenia or in dengue severe cases- [23]. Missing DENV in co-infection cases or misdiagnosis dengue fever as chikungunya may also delay or disrupt dengue-specific supportive treatment which can have a ten-fold impact on the likelihood of progression to severe disease [23], [24], [25]. For that, clinical management in dengue-endemic areas, recommends that patients with suspected CHIKV infection to be managed as dengue until dengue has been ruled out.
Conclusion
Co-infection cases of DENV and CHIKV are reported in India since 1964 [26], [27]. Co-circulation of all four DENV serotypes is confirmed since 1968 in South India [28]. In 2016, 111,880 DENV cases with 227 deaths and 58,136 clinically suspected CHIKV cases were reported in India [9], [29].
Curiously, although 98 countries/territories report both DENV and CHIKV epidemic/endemic transmission, the evidence of dengue and chikungunya co-infections has only been reported in 13 countries (Angola, Gabon, India, Madagascar, Malaysia, Myanmar, Nigeria, Saint Martin, Singapore, Sri Lanka, Tanzania, Thailand and Yemen) [22]. This is certainly weighed down by diagnosis mostly based on similar symptoms and the relative low rate of laboratory diagnosis confirmation in many, if not all endemic countries. Even though some studies on DENV and CHIKV co-infection were reported, there are still a lot to understand about the epidemiology of co-infection [22]. With the rapid geographic expansion of CHIKV to territories already endemic to DENV, the identification of synergistic or antagonistic pathogen interactions (including different serotypes and genotypes) within the vector and co-infected individuals is essential to assess the epidemiological consequences of DENV and CHIKV co-distribution [22].
Clinical awareness and laboratory confirmation of DENV suspected cases, with differential diagnosis with CHIKV (and eventually, Zika virus) and vice versa, is essential to detect co-infection cases and to assess the epidemiological understanding of these arboviral diseases, in endemic countries imported cases to non-endemic regions and its impact in morbidity and mortality.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contribution
LZZ: manuscript preparation and molecular diagnosis at INSA; COP: manuscript preparation, clinical data and laboratory findings at Infectious Diseases Unit, Matosinhos Local Health Unit; SJ, EP: clinical data and laboratory findings at Infectious Diseases Unit, Matosinhos Local Health Unit; LZZ, MJA: serological diagnosis at INSA; IN: head of Infectious Diseases Unit, Matosinhos Local Health Unit; MJA: laboratory coordination at INSA. All authors collaborated in the work and participated in the final revision of the manuscript.
Consent
Written consent was obtained from the patient and can be provided if requested, however no identifying information or images were used in this case report.
Conflicts of interest
The authors declare that there is no conflict of interests regarding the publication of this article.
Acknowledgements
Support from Biosystems and Integrative Sciences Institute (BioISI, FCT/UID/Multi/04046/2013) is acknowledged.
References
- 1.Halstead S. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239:476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- 2.WHO Dengue and severe dengue fact sheet. http://www.who.int/mediacentre/factsheets/fs117/en/ [Accessed 16 December 2016]
- 3.Bhatt S., Gething P.W., Brady O.J., Messina J.P., Farlow A.W., Moyes C.L. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brady O.J., Gething P.W., Bhatt S., Messina J.P., Brownstein J.S., Hoen A.G. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6:e1760. doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guzman M.G., Kouri G. Dengue: an update. Lancet Infect Dis. 2002;2:33–42. doi: 10.1016/s1473-3099(01)00171-2. [DOI] [PubMed] [Google Scholar]
- 6.Pialoux G., Gaüzère B.-A., Jauréguiberry S., Strobel M. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis. 2007;7:319–327. doi: 10.1016/S1473-3099(07)70107-X. [DOI] [PubMed] [Google Scholar]
- 7.Powers A.M., Brault A.C., Tesh R.B., Weaver S.C. Re-emergence of chikungunya and o’nyong-nyong viruses: evidence for distinct geographical lineages and distant evolutionary relationships. J Gen Virol. 2000;81:471–479. doi: 10.1099/0022-1317-81-2-471. [DOI] [PubMed] [Google Scholar]
- 8.Yergolkar P.N., Tandale B.V., Arankalle V.A., Sathe P.S., Sudeep A.B., Gandhe S.S. Chikungunya outbreaks caused by African genotype, India. Emerg Infect Dis. 2006;12:1580–1583. doi: 10.3201/eid1210.060529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Vector Borne Disease Control Programme (NVBDCP), Government of India. Clinically Suspected Chikungunya Fever Cases Since 2010, http://nvbdcp.gov.in/chik-cd.html [Accessed 24 January 2017].
- 10.Thiberville S.-D., Moyen N., Dupuis-Maguiraga L., Nougairede A., Gould E.A., Roques P. Chikungunya fever: epidemiology, clinical syndrome, pathogenesis and therapy. Antiviral Res. 2013;99:345–370. doi: 10.1016/j.antiviral.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez-Morales A.J., Gil-Restrepo A.F., Ramírez-Jaramillo V., Montoya-Arias C.P., Acevedo-Mendoza W.F., Bedoya-AriasJE Post-chikungunya chronic inflammatory rheumatism: results from a retrospective follow-up study of 283 adult and child cases in La Virginia, Risaralda, Colombia. F1000Res. 2016;5(360) doi: 10.12688/f1000research.8235.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alves M.J., Fernandes P.L., Amaro F., Osorio H., Luz T., Parreira P. Clinical presentation and laboratory findings for the first autochthonous cases of dengue fever in Madeira island, Portugal, October 2012. Euro Surveill. 2013;18(6) pii = 20398. [PubMed] [Google Scholar]
- 13.Parola P., de Lamballerie X., Jourdan J., Rovery C., Vaillant V., Minodier P. Novel chikungunya virus variant in travelers returning from Indian Ocean Islands. Emerg Infect Dis. 2006;12(10):1493–1499. doi: 10.3201/eid1210.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pastorino B., Bessaud M., Grandadam M., Muyri S., Tolou H.J., Peyrefitte C.N. Development of TaqMan® RT-PCR assay without RNA extraction step for the detection and quantification of African chikungunya viruses. J Virol Methods. 2005;124:65–71. doi: 10.1016/j.jviromet.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Lanciotti R.S., Calisher C.H., Gubler D.J., Chang G.J., Vorndam A.V. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polimerase chain reaction. J Clin Microbiol. 1992;30:545–551. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saxena P., Dash P.K., Santhosh S.R., Shrivastava A., Parida M., Lakshmana Rao P.V. Development and evaluation of one step single tube multiplex RT-PCR for rapid detection and typing of dengue viruses. Virol J. 2008;5:20. doi: 10.1186/1743-422X-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altschul S.F., Madden T.L., Schäffer A.A., Zhang J., Zhang Z., Miller W. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manakkadan A., Joseph I., Prasanna R.R., Kunju R.I., Kailas L., Sreekumar E. Lineage shift in Indian strains of dengue virus serotype-3 (Genotype III), evidenced by detection of lineage IV strains in clinical cases from Kerala. Virol J. 2013;10:37. doi: 10.1186/1743-422X-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shihada S., Emmerich P., Thomé-Bolduan C., Jansen S., Günther S., Frank C. Genetic diversity and new lineages of dengue virus serotypes 3 and 4 in returning travelers, Germany, 2006–2015. Emerg Infect Dis. 2017;23:272–275. doi: 10.3201/eid2302.160751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chevillon C., Briant L., Renaud F., Devaux C. The chikungunya threat: an ecological and evolutionary perspective. Trends Microbiol. 2008;16:80–88. doi: 10.1016/j.tim.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Furuya-Kanamori L., Liang S., Milinovich G., Magalhaes R.J. Soares, Clements A.C.A., Hu W. Co-distribution and co-infection of chikungunya and dengue viruses. BMC Infect Dis. 2016;16(84) doi: 10.1186/s12879-016-1417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laoprasopwattana K., Kaewjungwad L., Jarumanokul R., Geater A. Differential diagnosis of chikungunya, dengue viral infection and other acute febrile illnesses in children. Pediatr Infect Dis J. 2012;31:459–463. doi: 10.1097/INF.0b013e31824bb06d. [DOI] [PubMed] [Google Scholar]
- 24.Lam P.K., Tam D.T.H., Diet T.V., Tam C.T., Tien N.T.H., Kieu N.T.T. Clinical characteristics of dengue shock syndrome in Vietnamese children: a 10-year prospective study in a single hospital. Clin Infect Dis. 2013;57:1577–1586. doi: 10.1093/cid/cit594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomashek K.M., Biggerstaff B.J., Ramos M.M., Pérez-Guerra C.L., Rivera E.J. Garcia, Sun W. Physician survey to determine how dengue is diagnosed, treated and reported in Puerto Rico. PLoS Negl Trop Dis. 2014;8:e3192. doi: 10.1371/journal.pntd.0003192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myers R.M., Carey D.E. Concurrent isolation from patient of two arboviruses, chikungunya and dengue type 2. Science. 1967;157:1307–1308. doi: 10.1126/science.157.3794.1307. [DOI] [PubMed] [Google Scholar]
- 27.Carey D.E., Myers R.M., DeRanitz C.M., Jadhav M., Reuben R. The 1964 chikungunya epidemic at Vellore, South India, including observations on concurrent dengue. Trans R Soc Trop Med Hyg. 1969;63:434–435. doi: 10.1016/0035-9203(69)90030-3. [DOI] [PubMed] [Google Scholar]
- 28.Myers R.M., Varkey M.J., Reuben R., Jesudass E.S. Dengue outbreak in Vellore, southern India, in 1968, with isolation of four dengue types from man and mosquitoes. Indian J Med Res. 1970;58:24–30. [PubMed] [Google Scholar]
- 29.National Vector Borne Disease Control Programme (NVBDCP), Government of India. Dengue cases and deaths in the Countrysince 2010, http://nvbdcp.gov.in/den-cd.html [Accessed 24 January 2017].