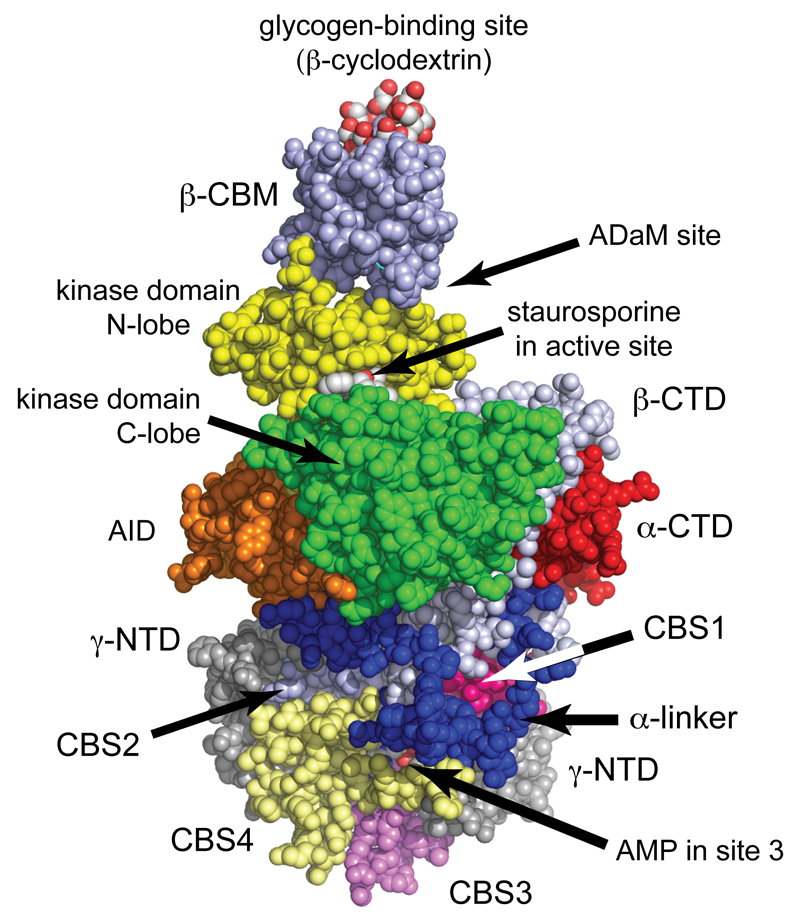
Figure 2. Structure of human AMPK (α1β2γ1 complex)7.
The model was created in spacefilling mode using PyMol v1.7.4.2 with the co-ordinates in PDB file 4RER, and with color coding similar to Fig. 1. The heterotrimer was crystallized in the presence of β-cyclodextrin, which occupies the glycogen-binding site, staurosporine, which occupies the active site, and AMP, which occupies sites 1, 3 and 4 on the γ subunit (sites 1 and 4 are round the back in this view). Although Thr172 was phosphorylated, it is not visible in this view but lies in the cleft between the α subunit C lobe and the β-CTD, just over the right-hand “shoulder” of the C-lobe.