Abstract
The intestinal milieu is astonishingly complex and home to a constantly changing mixture of small and large molecules, along with an abundance of bacteria, viral particles, and eukaryotic cells. Such complexity makes it difficult to develop testable molecular hypotheses regarding host-microbe interactions. Fortunately, mammals and their associated gastrointestinal (GI) microbes contain complementary systems that are ideally suited for mechanistic studies. Mammalian systems inactivate endobiotic and xenobiotic compounds by linking them to a glucuronic acid sugar for GI excretion. In the GI tract, the microbiota express β-glucuronidase enzymes that remove the glucuronic acid as a carbon source, effectively reversing the actions of mammalian inactivation. Thus, by probing the actions of microbial β-glucuronidases, and by understanding which substrate glucuronides they process, molecular insights into mammalian-microbial symbioses may be revealed amid the complexity of the intestinal tract. Here, we focus on glucuronides in the gut and the microbial proteins that process them.
Keywords: bacterial metabolism, drug metabolism, microbiome, symbiosis, xenobiotic, endobiotic, enterohepatic circulation
Introduction
β-Glucuronidase (GUS)2 enzymes expressed by the GI microbiota are at the interface of a metabolic symbiosis between microbe and host where they mediate the reactivation of molecules important in host health and disease. Microbial GUS enzymes regenerate toxic drugs and carcinogens in the mammalian GI tract (1), and their activities are associated with a higher incidence of colon cancer and to diets that promote intestinal cancer (2). Endogenous molecules are also processed by GI GUS proteins, including glucuronides of hormones and neurotransmitters (3–5). These observations have led to hypotheses linking microbial GUS enzymes to the GI toxicity of drugs, the development of cancer, and increased incidence of Crohn's disease and colitis (2, 6–9). Thus, bacterial GUS enzymes appear to play an important role in health and disease by metabolizing glucuronides in the gut.
GUS proteins catalyze the hydrolysis of glycosidic bonds between glucuronic acid and either small molecules or the terminal ends of polysaccharides. For the purposes of this review, we will focus on small molecule glucuronides generated by Phase II drug metabolism to mark compounds for excretion. Glucuronides are produced by mammalian uridine diphosphate (UDP)-glucuronosyltransferase (UGT) enzymes that append glucuronic acid, derived from UDP-glucuronate, to hydroxyl, carboxylate, and other nucleophilic functional groups of aglycones (10). Glucuronidation almost exclusively inactivates and detoxifies molecules by increasing their water solubility, which promotes their removal from the body via the kidneys or GI tract (11). Once in the GI tract, these glucuronides serve as substrates for bacterial GUS proteins that remove the inactivating glucuronic acid moiety. Glucuronic acid then enters the Entner-Doudoroff pathway, a bacterial alternative to glycolysis that catabolizes sugar acids and shunts the resulting pyruvate into the TCA cycle (12). Mammals also express a GUS enzyme ortholog that is localized to lysosomes in first-pass tissues like liver and intestines, and plays an essential role in degrading endogenous glycosaminoglycans (13). Germ line mutations in human GUS cause Sly syndrome, a fatal lysosomal storage disease (14). Human GUS has also been shown to hydrolyze small-molecule glucuronides, a function that has been leveraged in drug design by attaching drugs to glucuronic acid such that they will be activated at a site of interest upon hydrolysis (15).
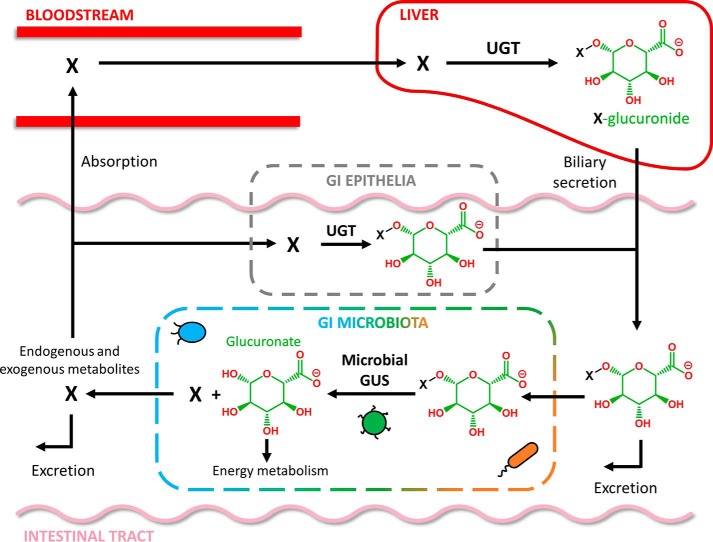
As a by-product of glucuronide hydrolysis, bacteria regenerate the original molecule that was eliminated by the host, facilitating reuptake by the gut epithelia and recirculation in the bloodstream (16). Glucuronidation in the liver, delivery to the GI lumen via the bile duct, reactivation and absorption via the intestinal epithelia, and transport back to the liver is termed enterohepatic circulation (Fig. 1) (17), and it can significantly affect the pharmacokinetics of many drugs and also regulates the levels of endogenous compounds (4, 17, 18). Thus, GI microbial GUS enzymes have the capability of directly regulating local and systemic levels of exogenous and endogenous compounds involved in mammalian homeostasis.
Figure 1.
Enterohepatic circulation of chemically distinct molecules (denoted as X) is mediated by the host and microbiota. Glucuronides (e.g. X-glucuronide) are generated primarily in the liver by UGTs (but can also be produced by GI epithelial UGTs) and then delivered by biliary secretion to the GI. In the alimentary canal, glucuronides are either excreted or metabolized by the GI microbiota's GUS enzymes. Reactivated aglycones in the GI (X) can be excreted or reabsorbed and returned to the liver via the enterohepatic cycle.
Endogenous glucuronides in the gut
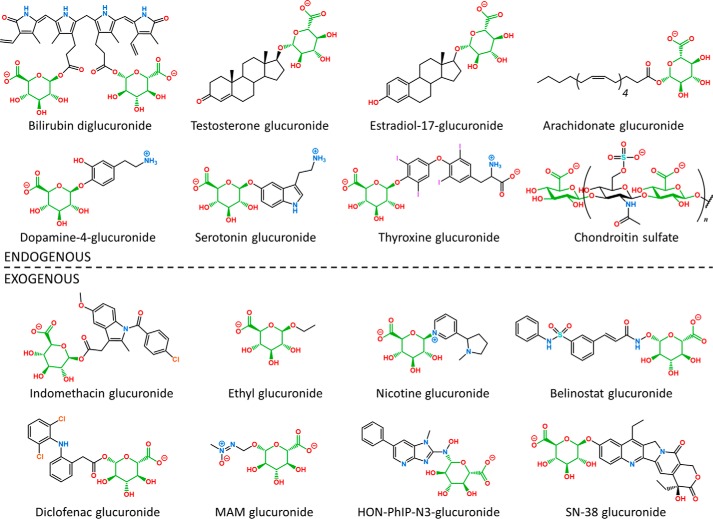
Endogenous glucuronides were clearly the driving force for the symbiotic evolution of host-associated bacterial GUS enzymes. Glucuronidated endogenous compounds include bilirubin, hormones, neurotransmitters, bile acids, and fatty acids, all of which influence host homeostasis (Table 1 and Fig. 2). As such, GI microbial GUS enzymes participate in a nearly constant mutual symbiosis via the regulation of local and systemic levels of endogenous molecules.
Table 1.
Examples of molecules subject to glucuronidation in mammals
IBD is inflammatory bowel disease.
| Aglycone | Aglycone's effect | Disease/health | Refs. |
|---|---|---|---|
| Endogenous | |||
| Arachidonic acid | Signaling molecule | Inflammation | 48 |
| Bilirubin | Neurotoxin, antioxidant | Gall stones, jaundice | 19 |
| Chenodeoxycholate | Digestion | Cholestasis | 89 |
| Chondroitin sulfate | Glycosaminoglycan | Cancer | 90 |
| Dopamine | GI motility, water absorption | IBD, constipation | 5 |
| Estradiol | Sex hormone, development | Breast cancer | 26 |
| Hyaluronic acid | Glycosaminoglycan | Cancer | 10 |
| Norepinephrine | GI motility | IBD | 5 |
| Serotonin | GI motility | IBD | 40 |
| Testosterone | Sex hormone | Prostate cancer | 32 |
| Thyroxine | Thyroid regulation | Metabolic disorder | 36 |
| Exogenous | |||
| AOM | Alkylating agent | Cancer | 66 |
| Belinostat | HDAC inhibitor | GI toxicity | 57 |
| Benzo[a]pyrene | DNA adduct formation | Cancer | 70 |
| Diclofenac | NSAID | GI toxicity | 62 |
| Ethanol | Depressant | Liver toxicity | 91 |
| Indomethacin | NSAID | GI toxicity | 64 |
| Ketoprofen | NSAID | GI toxicity | 62 |
| Nicotine | Stimulant | Addiction | 75 |
| Panobinostat | HDAC inhibitor | GI toxicity | 58 |
| PhIP | Alkylating agent | Cancer | 69 |
| SN-38 | Topoisomerase I inhibitor | GI toxicity | 54 |
Figure 2.
Examples of chemically diverse endogenous and exogenous glucuronides (glucuronic acid shown in green) generated by mammalian UGT enzymes and metabolized by GI microbial GUS enzymes.
One of the most heavily glucuronidated endogenous molecules is bilirubin, a breakdown product of heme (19). Although it is generally considered a waste product and toxin that contributes to hyperbilirubinemia and neonatal jaundice, normal levels of bilirubin have more recently been shown to have preventative antioxidant activities (20, 21). Approximately 16 and 80% of bilirubin exists as the monoglucuronide and diglucuronide conjugates, respectively, in the bile of healthy humans (19). Bilirubin glucuronides are generated in the liver by UGT1A1 and enter the GI tract from the bile duct. In the GI tract, bilirubin glucuronides are heavily metabolized by the intestinal microbiota into stercobilin, which gives feces its brown color, and urobilin, which is responsible for the yellow color of urine and the yellow complexion of jaundiced subjects (22). The deconjugated bilirubin that manages to escape further metabolism by bacteria is reabsorbed through the GI epithelia and undergoes enterohepatic circulation (18). However, enterohepatic circulation of bilirubin in healthy humans is relatively low due to bilirubin's nearly complete glucuronidation by the host and substantial subsequent metabolism to stercobilin and urobilin by the GI microbiota. In certain neonates or subjects with Gilbert's syndrome, however, bilirubin is significantly recycled, which contributes to CNS-toxic hyperbilirubinemia (19, 20). Thus, bacterial GUS and human UGT enzymes appear to have co-evolved a mutually symbiotic heme catabolism pathway to rid the host of high levels of toxic bilirubin and to provide the GI microbiota with a source of energy in the form of glucuronic acid.
Unconjugated bilirubin is also capable of forming insoluble calcium salts that contribute to the generation of brown pigment stones in the gallbladder and the biliary ductal system, which reduce bile flow and can impair liver function (23). Interestingly, the generation of these stones is concomitant with the presence of GUS-expressing Proteobacteria like Escherichia coli and Klebsiella pneumoniae, suggesting that bacterial GUS activity may promote the formation of the unconjugated bilirubin salts found in gallstones (23, 24). Bacteria of the family Enterobacteriaceae, which include E. coli and K. pneumoniae, are more abundant in the bile (25). The low affinity GUS inhibitor glucaro-1,4-lactone blocked calcium bilirubinate precipitation in vitro (23).
Hormones are also subject to glucuronidation. The estrogen metabolites estradiol, estrone, and estriol are glucuronidated by multiple UGT isoforms (26). In vitro studies have shown that E. coli GUS is capable of hydrolyzing a glucuronide metabolite of estriol, and it does so with much greater activity than human GUS (27). Furthermore, estrogen metabolites exhibit significant enterohepatic circulation, suggesting that the regeneration of estrogen aglycones by the GI microbiota may play an important role in regulating plasma levels of this hormone (28). Radiolabeling studies reveal that enterohepatic circulation of estrone and estriol varies by host species, which suggests that species differences in UGT expression or microbial composition may impact hormone metabolism (29). Although bacterial GUS has been demonstrated in vitro to hydrolyze estrogenic glucuronides, a definitive role for the GI microbiota in the enterohepatic circulation of estrogenic metabolites in vivo has not been established. However, as posited recently, the reactivation of estrogenic metabolites by the GI microbiota may promote the enterohepatic circulation of estrogenic metabolites, which may subsequently foster the growth of estrogen-responsive tumors (30). It is important to note, however, that estrogen metabolites are also heavily sulfated through the action of mammalian sulfotransferases, another set of Phase 2 drug-metabolizing enzymes that perform a role analogous to the glucuronidating UGTs (26). GI bacteria also harbor a variety of sulfatases to process highly sulfated polysaccharides and sulfated small molecules, including estrogen metabolites (26, 31). Overall, mammalian hormone inactivation is likely closely mirrored and reversed by enzymes in the GI microbiota.
Other glucuronidated hormones include the androgen testosterone and the thyroid hormone thyroxine (32, 33). Both androgen and thyroxine glucuronides can be hydrolyzed by bacterial GUS enzymes (27, 34, 35). Androgens are key drivers of prostate cancer, resulting in therapies primarily focused on androgen deprivation in the form of surgical or chemical castration, although a more recent approach is the enhancement of androgen glucuronidation by UGTs (7). Thyroxine is a primary thyroid hormone that impacts a variety of processes, including metabolic regulation (36). In vivo radiolabeling and ex vivo fecal assays indicate that bacteria play a key role in the enterohepatic circulation of thyroxine in mammals (34). As such, GI microbial GUS proteins could participate in the regulation of metabolism and development by promoting the enterohepatic circulation of thyroxine.
The neurotransmitters dopamine, norepinephrine, and serotonin are glucuronidated in the body and metabolized by bacterial GUS. Roughly 50% of all dopamine is generated in the GI tract (37), where it acts as a regulator of gut motility and water absorption (38, 39). Microbes were recently shown to have a significant role in the processing of dopamine glucuronide in the GI lumen of mice (5). This study utilized germ-free mouse models and GUS knock-out strains of bacteria to demonstrate that microbial GUS activity is primarily responsible for dopamine glucuronide hydrolysis. The neurotransmitter norepinephrine, a chemical cousin of dopamine, is also glucuronidated and exhibited microbe-mediated glucuronide hydrolysis in the GI lumen (5). Similarly, serotonin is subject to glucuronidation, and plasma levels of serotonin in mice fluctuate based on the presence or absence of the microbiota (40, 41).
Bile acids are important to gut health and are significantly processed by the microbiota. Bile acids are primarily considered detergents that solubilize dietary components for digestion (42). Much like bilirubin, bile acids are heavily metabolized by the microbiota, which can dehydrate, oxidize, and deconjugate bile acid variants generated by the liver (43). In the liver, bile acids are conjugated to sulfate, taurine, and glycine moieties, all of which can be removed by GI microbial sulfatases and bile salt hydrolases. Bile acids are also glucuronidated in the liver (44), and the resulting conjugates account for between 12 and 36% of the bile acids excreted in the urine. By contrast, sulfate, glycine, and taurine conjugates make up 50–63, 1.8–28, and 4.1–8.3% of excreted bile acids in the urine, respectively (45). Thus, glucuronidated bile acids likely provide a significant energy source to bacteria capable of processing such compounds. Unraveling the connections between host and microbial bile acid metabolism pathways will likely reveal new insights into the co-evolution of mammals and microbes.
Fatty acids are another class of biological detergents processed by liver UGTs. Fatty acids play roles in mammalian biology that range from cell signaling to membrane integrity (46). Ex vivo and in vitro analyses show that a variety of fatty acids can be glucuronidated, including arachidonic acid, retinoic acid, prostaglandins, and derivatives of linoleic acid (47–49), although further studies are needed to determine whether fatty acid glucuronides are processed by bacterial GUS enzymes.
Finally, endogenous polysaccharides are a critical source of glucuronides in the gut. Chondroitin sulfate and hyaluronic acid are glucuronic acid-containing polysaccharides present in the GI tract (10). Bacteria express a wealth of endo- and exo-glycosidases that work in concert to break down complex polysaccharides. Analogous to human GUS, which catabolizes extracellular matrix polysaccharides in lysosomes, bacterial GI GUS enzymes play similar roles with substrates like chondroitin sulfate that enter the GI from host cells sloughed from the epithelia (50). An excellent review of microbial polysaccharide-processing enzymes in the mammalian GI tract has recently been provided (51).
Exogenous glucuronides in the gut
Glucuronides of drugs and other exogenous molecules have been a primary focus of research because of their potential importance to therapeutic efficacy and tolerance. Many drugs exhibit GI and liver toxicity that is mediated in part by bacterial GUS activity in the gut, resulting in a parasitic symbiosis in which bacteria receive sugar from drug glucuronides and the host retains toxic metabolites. Carcinogens and other dietary metabolites are also metabolized in the body via glucuronidation and processed by our microbial counterparts, providing a link between bacterial GUS enzymes and carcinogenesis. Exogenous glucuronides that reach the GI are diverse in chemical structure, suggesting that a proportional breadth of functional diversity may be present in the collection of microbial GUS enzymes in the GI (Table 1 and Fig. 2).
The anticancer agent SN-38 is the archetype of how metabolism by bacterial GUS can lead to drug toxicity. SN-38 is the active form of the prodrug irinotecan, which is commonly used to treat colorectal and pancreatic cancers (52, 53). SN-38 is inactivated in the liver by conversion to SN-38-glucuronide (SN-38-G); in the GI lumen, however, microbial GUS enzymes recreate SN-38 and cause severe GI toxicity in the form of dose-limiting diarrhea. The authors' laboratory showed that potent, selective, and non-lethal inhibition of bacterial GUS enzymes reduces the GI toxicity of SN-38 in mice (54–56). This approach may improve the efficacy and tolerance of other anticancer drugs. Indeed, from a list of 155 anticancer agents, 24 are known to be glucuronidated, and of those that are glucuronidated, 21 (89% of 24) cause GI toxicity. Two such drugs are the histone deacetylase (HDAC) inhibitors belinostat and panobinostat used to treat lymphoma (57, 58). Metabolites of lapatinib, a GI toxic drug used to treat hormone receptor-positive breast cancer, are glucuronidated, and their reactivation may damage the liver as well as the GI tract (59). GI microbial GUS enzymes contribute to hepatotoxicity via the enhancement of enterohepatic circulation, which leads to repeated liver exposure to toxic metabolites (17). Together, these examples highlight the role that bacterial GUS plays in cancer treatment, efficacy, and toxicity.
Non-steroidal anti-inflammatory drugs (NSAIDs), some of the most widely used therapeutics in the world, are also glucuronidated. NSAIDs inhibit cyclooxygenase enzymes and prostaglandin synthesis and contain a carboxylic acid group that is readily glucuronidated (60). The NSAID diclofenac is conjugated to glucuronic acid by UGT2B7 in the liver, delivered to the GI tract via the bile duct, and hydrolyzed by bacterial GUS enzymes in the GI (61, 62). The regeneration of diclofenac causes ulceration of the GI epithelia via an unclear mechanism that may involve disruption of mitochondrial function (63). Similar to SN-38, prevention of diclofenac regeneration by a selective bacterial GUS inhibitor reduced GI ulceration in mice (61). The GI damage of the NSAIDs ketoprofen and indomethacin can also be ameliorated by selective inhibition of bacterial GUS (62). Interestingly, the GI toxicity caused by NSAIDs is primarily localized in mice to the distal end of the small intestine, whereas the damage most often associated with irinotecan is located in the large intestine (64). It is possible that the bacteria that thrive in the distal small intestine may have a greater capability to hydrolyze NSAID glucuronides than microbes in the proximal small intestine and colon (64).
Certain carcinogens are also glucuronidated. One of the most potent is the alkylating agent methylazoxymethanol (MAM), the active metabolite of azoxymethanol (AOM) that is used to model carcinogenesis in rodents (65). AOM is converted by cytochrome P450 2E1 and UGTs in the liver to generate MAM-glucuronide (MAM-G), and evidence exists that bacteria in the GI tract reactivate MAM-G to MAM and promote colon carcinogenesis (66, 67). The low-affinity bacterial GUS inhibitor C-GAL has been shown to reduce colon carcinogenesis caused by AOM (68). Other carcinogens, like the polyaromatic hydrocarbons and heterocyclic aromatic amines, are also metabolized by the CYP-to-UGT pathway, and it has been suggested that microbes hydrolyze those glucuronide metabolites as well (69, 70). Interestingly, colon cancer patients exhibit higher fecal GUS activities than controls (2). Together, these results support the conclusion that the release of active carcinogens in the GI tract involves microbial GUS enzymes.
Two widely used lifestyle drugs metabolized by host UGTs and bacterial GUSs are ethanol and nicotine. Although the majority of ingested ethanol is converted to acetaldehyde by alcohol dehydrogenase, a small fraction of ethanol is glucuronidated (71). In humans, ethanol glucuronide has been detected in the liver, bile, and urine (72). E. coli and Clostridum sordellii have both been shown to hydrolyze ethyl glucuronide in vitro, which may contribute to a greater retention of ethanol-derived metabolites in the body (73). Detection of ethyl glucuronide in hair has been employed as a biomarker to diagnose alcohol abuse (74). Nicotine and its metabolites are primarily processed in humans by oxidation, but they are also glucuronidated (75). Nicotine is unique among the aglycones discussed here in that it is conjugated to glucuronic acid through a nitrogen linkage, and microbial GUS enzymes have been shown to cleave nicotine glucuronide (76). The glucuronides of ethanol and nicotine highlight the chemical diversity of exogenous compounds that serve as substrates for GUS proteins of the GI microbiota.
Although not the primary focus of this review, a small number of plant polysaccharides that contain glucuronic acid are mentioned here. Gum Arabic is a plant-derived secretion that is predominantly composed of glucuronic acid-containing polysaccharides, and it is widely utilized in the food and drug industry as a stabilizer (10, 77). This complex polysaccharide is indigestible to animals but can be fermented by bacteria in the colon and is associated with weight loss in humans (78). The xylan hemicelluloses, which are heteropolymers of various sugars and components of the plant cell wall, also contain glucuronic acid (10). Like Gum Arabic, xylan polysaccharides are indigestible by human enzymes but can be catabolized by GI microbes. Xylan complexity appears to require a diverse set of microbial xylanases to catabolize them to release smaller glucuronic acid-containing sugars further processed by intestinal bacteria (79).
Microbial β-glucuronidases in the gut
Several investigations have detected in vitro GUS activity, ex vivo fecal GUS activity, and in vivo correlations between GI GUS enzyme activity and health. These studies have resulted in the identification of bacteria related to Crohn's disease, the discovery of increased GUS activity in patients with colorectal cancer and subjects on high fat diets, and the mechanistic elucidation of how bacterial GUS promotes drug toxicity (1, 2, 9, 55). To test the relationship between microbial GUS activity and disease, total fecal proteins have been extracted and GUS assays conducted (80). This approach yields an overall view of the fecal microbiota's GUS activity but provides little granularity about the specific microbial enzymes involved. Other approaches involve culturing bacteria obtained from human fecal samples and then assessing the GUS activity in these pure cultures (6, 81–88). A tabulation of strains analyzed in culture-based GUS activity assays reveals that bacteria from all the major phyla in the mammalian GI microbiota, including Actinobacteria, Bacteroidetes, Firmicutes, and Proteobacteria, harbor enzymes that process glucuronides (Table 2). The conservation of GUS across all major GI bacterial phyla reinforces the hypothesis that GUS proteins may play key roles in chemical dynamics across the intestinal epithelium and serve as a competitive growth advantage for bacteria in the crowded and unforgiving milieu of the mammalian gut.
Table 2.
Bacterial strains from the human microbiota that have been shown to exhibit GUS activity in culture
| Strain | Refs. |
|---|---|
| Actinobacteria | |
| Bifidobacterium adolescentis JCM 1275 | 87 |
| Bifidobacterium angulatum NCFB 2237 | 86 |
| Bifidobacterium bifidum NCFB 2454 | 86 |
| Bifidobacterium breve NCFB 2257 | 86 |
| Bifidobacterium longum JCM 1217 | 87 |
| Bifidobacterium pseudolongum NCFB 2244 | 86 |
| Collinsella aerofaciens JCM 7790 | 87 |
| Bacteroidetes | |
| Bacteroides capillosus ATCC 29799 | 84 |
| Bacteroides fragilis NCFB 2217 | 86 |
| Bacteroides ovatus ATCC 8483 | 84 |
| Bacteroides thetaiotaomicron | 87 |
| Bacteroides uniformis JCM 5828 | 87 |
| Bacteroides vulgatus DCNC 23 | 86 |
| Parabacteroides johnsonii DSM 18315 | 84 |
| Parabacteroides merdae ATCC 43184 | 84 |
| Firmicutes | |
| Bryantella formatexigens DSM 14469 | 84 |
| Clostridium bartlettii DSM 16795 | 84 |
| Clostridium bifermentans NCFB 2189 | 86 |
| Clostridium butyricum DCNC 19 | 86 |
| Clostridium clostridioforme JCM 1291 | 87 |
| Clostridium paraputrificum JCM 1293 | 87 |
| Clostridium perfringens NCTC 8679 | 86 |
| Enterococcus faecalis DCNC 24 | 86 |
| Enterococcus faecium DCNC 26 | 86 |
| Eubacterium L-8 | 85 |
| Faecalibacterium prausnitzii M21/2 | 84 |
| Lactobacillus acidophilus DCNC 1237 | 86 |
| Lactobacillus gasseri ADH | 88 |
| Roseburia inulinivorans DSM 16841 | 84 |
| Ruminococcus gnavus ATCC 29149 | 84 |
| Ruminococcus gnavus E1 | 81 |
| Subdoligranulum variabile DSM 15176 | 84 |
| Streptococcus LJ-22 | 85 |
| Proteobacteria | |
| E. coli HGU-3 | 6 |
Acknowledgments
We thank Rebecca Pollet, Aadra Bhatt, William Walton, Michael Little, Kristen Biernat, and Ben Creekmore for considerable intellectual assistance.
This work was supported by National Institutes of Health Grants CA207416 and CA098468 and Cellular and Molecular Biophysics Program of University of North Carolina Grant 5T32GM008570-20. This is the fourth article in the Host-Microbiome metabolic interplay Minireview series. The authors of this publication have equity ownership (M. R. R.) or are inventors of technologies (S. J. P. and M. R. R.) related to Symberix, Inc., a pharmaceutical company creating microbiome-targeted therapeutics. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- GUS
- β-glucuronidase
- GI
- gastrointestinal
- UGT
- UDP-glucuronosyltransferase
- NSAID
- non-steroidal anti-inflammatory drugs
- MAM
- methylazoxymethanol
- MAM-G
- MAM-glucuronide
- AOM
- azoxymethanol
- HDAC
- histone deacetylase.
References
- 1. Goldin B. R. (1986) In situ bacterial metabolism and colon mutagens. Annu. Rev. Microbiol. 40, 367–393 [DOI] [PubMed] [Google Scholar]
- 2. Goldin B. R., and Gorbach S. L. (1976) The relationship between diet and rat fecal bacterial enzymes implicated in colon cancer. J. Natl. Cancer Inst. 57, 371–375 [DOI] [PubMed] [Google Scholar]
- 3. Lombardi P., Goldin B., Boutin E., and Gorbach S. L. (1978) Metabolism of androgens and estrogens by human fecal microorganisms. J. Steroid Biochem. 9, 795–801 [DOI] [PubMed] [Google Scholar]
- 4. Winter J., and Bokkenheuser V. D. (1987) Bacterial metabolism of natural and synthetic sex hormones undergoing enterohepatic circulation. J. Steroid Biochem. 27, 1145–1149 [DOI] [PubMed] [Google Scholar]
- 5. Asano Y., Hiramoto T., Nishino R., Aiba Y., Kimura T., Yoshihara K., Koga Y., and Sudo N. (2012) Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G1288–G1295 [DOI] [PubMed] [Google Scholar]
- 6. Kim D. H., and Jin Y. H. (2001) Intestinal bacterial β-glucuronidase activity of patients with colon cancer. Arch. Pharm. Res. 24, 564–567 [DOI] [PubMed] [Google Scholar]
- 7. Grosse L., Pâquet S., Caron P., Fazli L., Rennie P. S., Bélanger A., and Barbier O. (2013) Androgen glucuronidation: an unexpected target for androgen deprivation therapy, with prognosis and diagnostic implications. Cancer Res. 73, 6963–6971 [DOI] [PubMed] [Google Scholar]
- 8. Plotnikoff G. A. (2014) Three measurable and modifiable enteric microbial biotransformations relevant to cancer prevention and treatment. Glob. Adv. Health Med. 3, 33–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gloux K., and Anba-Mondoloni J. (2016) Unique β-glucuronidase locus in gut microbiomes of Crohn's disease patients and unaffected first-degree relatives. PLoS ONE 11, e0148291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dutton G. J. (ed) (1966) Glucuronic Acid, Free and Combined, Biochemistry, Pharmacology, and Medicine. Academic Press, Orlando, FL [Google Scholar]
- 11. Dutton G. J. (ed) (1980) Glucuronidation of Drugs and Other Compounds, pp. 69–74, CRC Press, Inc., Boca Raton, FL [Google Scholar]
- 12. Peekhaus N., and Conway T. (1998) What's for dinner?: Entner-Doudoroff metabolism in Escherichia coli. J. Bacteriol. 180, 3495–3502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jain S., Drendel W. B., Chen Z. W., Mathews F. S., Sly W. S., and Grubb J. H. (1996) Structure of human β-glucuronidase reveals candidate lysosomal targeting and active-site motifs. Nat. Struct. Biol. 3, 375–381 [DOI] [PubMed] [Google Scholar]
- 14. Sly W. S., Quinton B. A., McAlister W. H., and Rimoin D. L. (1973) β-Glucuronidase deficiency: report of clinical, radiologic, and biochemical features of a new mucopolysaccharidosis. J. Pediatr. 82, 249–257 [DOI] [PubMed] [Google Scholar]
- 15. Sperker B., Backman J. T., and Kroemer H. K. (1997) The role of β-glucuronidase in drug disposition and drug targeting in humans. Clin. Pharmacokinet. 33, 18–31 [DOI] [PubMed] [Google Scholar]
- 16. Wilson K. J., Hughes S. G., and Jefferson R. (1992) in The Escherichia coli gus Operon: Induction and Expression of the gus Operon in E. coli and the Occurrence and Use of GUS in Other Bacteria (Gallagher S. R., ed) pp. 7–22, Academic Press, Inc., San Diego [Google Scholar]
- 17. Roberts M. S., Magnusson B. M., Burczynski F. J., and Weiss M. (2002) Enterohepatic circulation: physiological, pharmacokinetic and clinical implications. Clin. Pharmacokinet. 41, 751–790 [DOI] [PubMed] [Google Scholar]
- 18. Vítek L., and Carey M. C. (2003) Enterohepatic cycling of bilirubin as a cause of “black” pigment gallstones in adult life. Eur. J. Clin. Invest. 33, 799–810 [DOI] [PubMed] [Google Scholar]
- 19. Fevery J., Blanckaert N., Leroy P., Michiels R., and Heirwegh K. P. (1983) Analysis of bilirubins in biological fluids by extraction and thin-layer chromatography of the intact tetrapyrroles: application to bile of patients with Gilbert's syndrome, hemolysis, or cholelithiasis. Hepatology 3, 177–183 [DOI] [PubMed] [Google Scholar]
- 20. Vítek L., Kotal P., Jirsa M., Malina J., Cerná M., Chmelar D., and Fevery J. (2000) Intestinal colonization leading to fecal urobilinoid excretion may play a role in the pathogenesis of neonatal jaundice. J. Pediatr. Gastroenterol. Nutr. 30, 294–298 [DOI] [PubMed] [Google Scholar]
- 21. Stocker R., Yamamoto Y., McDonagh A. F., Glazer A. N., and Ames B. N. (1987) Bilirubin is an antioxidant of possible physiological importance. Science. 235, 1043–1046 [DOI] [PubMed] [Google Scholar]
- 22. Vítek L., and Ostrow J. D. (2009) Bilirubin chemistry and metabolism; harmful and protective aspects. Curr. Pharm. Des. 15, 2869–2883 [DOI] [PubMed] [Google Scholar]
- 23. Maki T. (1966) Pathogenesis of calcium bilirubinate gallstone: role of E. coli, β-glucuronidase and coagulation by inorganic ions, polyelectrolytes and agitation. Ann. Surg. 164, 90–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shen H., Ye F., Xie L., Yang J., Li Z., Xu P., Meng F., Li L., Chen Y., Bo X., Ni M., and Zhang X. (2015) Metagenomic sequencing of bile from gallstone patients to identify different microbial community patterns and novel biliary bacteria. Sci. Rep. 5, 17450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ye F., Shen H., Li Z., Meng F., Li L., Yang J., Chen Y., Bo X., Zhang X., and Ni M. (2016) Influence of the biliary system on biliary bacteria revealed by bacterial communities of the human biliary and upper digestive tracts. PLoS ONE 11, e0150519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Raftogianis R., Creveling C., Weinshilboum R., and Weisz J. (2000) Estrogen metabolism by conjugation. J. Natl. Cancer Inst. Monogr. 2000, 113–124 [DOI] [PubMed] [Google Scholar]
- 27. Graef V., Furuya E., and Nishikaze O. (1977) Hydrolysis of steroid glucuronides with β-glucuronidase preparations from bovine liver, helix pomatia, and E. coli. Clin. Chem. 23, 532–535 [PubMed] [Google Scholar]
- 28. Sher A., and Rahman M. A. (2000) Enterohepatic recycling of estrogen and its relevance with female fertility. Arch. Pharm. Res. 23, 513–517 [DOI] [PubMed] [Google Scholar]
- 29. Sandberg A. A., Kirdani R. Y., Back N., Weyman P., and Slaunwhite W. R. (1967) Biliary excretion and enterohepatic circulation of estrone and estriol in rodents. Am. J. Physiol. 213, 1138–1142 [DOI] [PubMed] [Google Scholar]
- 30. Kwa M., Plottel C. S., Blaser M. J., and Adams S. (2016) The intestinal microbiome and estrogen receptor–positive female breast cancer. J. Natl. Cancer Inst. 108, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ulmer J. E., Vilén E. M., Namburi R. B., Benjdia A., Beneteau J., Malleron A., Bonnaffé D., Driguez P. A., Descroix K., Lassalle G., Le Narvor C., Sandström C., Spillmann D., and Berteau O. (2014) Characterization of glycosaminoglycan (GAG) sulfatases from the human gut symbiont Bacteroides thetaiotaomicron reveals the first GAG-specific bacterial endosulfatase. J. Biol. Chem. 289, 24289–24303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kreek M. J., Guggenheim F. G., Ross J. E., and Tapley D. F. (1963) Glucuronide formation in the transport of testosterone and androstenedione by rat intestine. Biochim. Biophys. Acta 74, 418–427 [DOI] [PubMed] [Google Scholar]
- 33. Yamanaka H., Nakajima M., Katoh M., and Yokoi T. (2007) Glucuronidation of thyroxine in human liver, jejunum, and kidney microsomes. Drug Metab. Dispos. 35, 1642–1648 [DOI] [PubMed] [Google Scholar]
- 34. Hazenberg M. P., de Herder W. W., and Visser T. J. (1988) Hydrolysis of iodothyronine conjugates by intestinal bacteria. FEMS Microbiol. Rev. 4, 9–16 [DOI] [PubMed] [Google Scholar]
- 35. DiStefano J. J. 3rd., de Luze A., and Nguyen T. T. (1993) Binding and degradation of 3,5,3′-triiodothyronine and thyroxine by rat intestinal bacteria. Am. J. Physiol. 264, E966–E972 [DOI] [PubMed] [Google Scholar]
- 36. Zhang J., and Lazar M. (2000) The mechanism of action of thyroid hormones. Annu. Rev. Physiol. 62, 439–66 [DOI] [PubMed] [Google Scholar]
- 37. Eisenhofer G., Aneman A., Friberg P., Hooper D., Fåndriks L., Lonroth H., Hunyady B., and Mezey E. (1997) Substantial production of dopamine in the human gastrointestinal tract. J. Clin. Endocrinol. Metab. 82, 3864–3871 [DOI] [PubMed] [Google Scholar]
- 38. Flemström G., Säfsten B., and Jedstedt G. (1993) Stimulation of mucosal alkaline secretion in rat duodenum by dopamine and dopaminergic compounds. Gastroenterology. 104, 825–833 [DOI] [PubMed] [Google Scholar]
- 39. Haskel Y., and Hanani M. (1994) Inhibition of gastrointestinal motility by MPTP via adrenergic and dopaminergic mechanisms. Dig. Dis. Sci. 39, 2364–2367 [DOI] [PubMed] [Google Scholar]
- 40. Krishnaswamy S., Duan S. X., Von Moltke L. L., Greenblatt D. J., Sudmeier J. L., Bachovchin W. W., and Court M. H. (2003) Serotonin (5-hydroxytryptamine) glucuronidation in vitro: assay development, human liver microsome activities and species differences. Xenobiotica 33, 169–180 [DOI] [PubMed] [Google Scholar]
- 41. Wikoff W. R., Anfora A. T., Liu J., Schultz P. G., Lesley S. A., Peters E. C., and Siuzdak G. (2009) Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. U.S.A. 106, 3698–3703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hofmann A. F. (2009) The enterohepatic circulation of bile acids in mammals: form and functions. Front. Biosci. 14, 2584–2598 [DOI] [PubMed] [Google Scholar]
- 43. Devlin A. S., and Fischbach M. A. (2015) A biosynthetic pathway for a prominent class of microbiota-derived bile acids. Nat. Chem. Biol. 11, 685–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Matern S., Matern H., Farthmann E. H., and Gerok W. (1984) Hepatic and extrahepatic glucuronidation of bile acids in man. Characterization of bile acid uridine 5′-diphosphate-glucuronosyltransferase in hepatic, renal, and intestinal microsomes. J. Clin. Invest. 74, 402–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Almé B., and Sjövall J. (1980) Analysis of bile acid glucuronides in urine. identification of 3α,6α,12α-trihydroxy-5β-cholanoic acid. J. Steroid Biochem. 13, 907–916 [DOI] [PubMed] [Google Scholar]
- 46. Kremmyda L.-S., Tvrzicka E., Stankova B., and Zak A. (2011) Fatty acids as biocompounds: their role in human metabolism, health and disease–a review. Part 2: fatty acid physiological roles and applications in human health and disease. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 155, 195–218 [DOI] [PubMed] [Google Scholar]
- 47. Prakash C., Zhang J. Y., Falck J. R., Chauhan K., and Blair I. A. (1992) 20-Hydroxyeicosatetraenoic acid is excreted as a glucuronide conjugate in human urine. Biochem. Biophys. Res. Commun. 185, 728–733 [DOI] [PubMed] [Google Scholar]
- 48. Barua A. B., and Olson J. A. (1986) Retinoyl β-glucuronide: an endogenous compound of human blood. Am. J. Clin. Nutr. 43, 481–485 [DOI] [PubMed] [Google Scholar]
- 49. Little J. M., Kurkela M., Sonka J., Jäntti S., Ketola R., Bratton S., Finel M., and Radominska-Pandya A. (2004) Glucuronidation of oxidized fatty acids and prostaglandins B1 and E2 by human hepatic and recombinant UDP-glucuronosyltransferases. J. Lipid Res. 45, 1694–1703 [DOI] [PubMed] [Google Scholar]
- 50. Salyers A. A., and O'Brien M. (1980) Cellular location of enzymes involved in chondroitin sulfate breakdown by Bacteroides thetaiotaomicron. J. Bacteriol. 143, 772–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Koropatkin N. M., Cameron E. A., and Martens E. C. (2012) How glycan metabolism shapes the human gut microbiota. Nat. Rev. Microbiol. 10, 323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pommier Y. (2006) Topoisomerase I inhibitors: camptothecins and beyond. Nat. Rev. Cancer 6, 789–802 [DOI] [PubMed] [Google Scholar]
- 53. Cantore M., Rabbi C., Fiorentini G., Oliani C., Zamagni D., Iacono C., Mambrini A., Del Freo A., and Manni A. (2004) Combined irinotecan and oxaliplatin in patients with advanced pre-treated pancreatic cancer. Oncology 67, 93–97 [DOI] [PubMed] [Google Scholar]
- 54. Robert J., and Rivory L. (1998) Pharmacology of irinotecan. Drugs Today 34, 777–803 [DOI] [PubMed] [Google Scholar]
- 55. Wallace B. D., Wang H., Lane K. T., Scott J. E., Orans J., Koo J. S., Venkatesh M., Jobin C., Yeh L.-A., Mani S., and Redinbo M. R. (2010) Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 330, 831–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wallace B. D., Roberts A. B., Pollet R. M., Ingle J. D., Biernat K. A., Pellock S. J., Venkatesh M. K., Guthrie L., O'Neal S. K., Robinson S. J., Dollinger M., Figueroa E., McShane S. R., Cohen R. D., Jin J., et al. (2015) Structure and inhibition of microbiome β-glucuronidases essential to the alleviation of cancer drug toxicity. Chem. Biol. 22, 1238–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang L. Z., Ramírez J., Yeo W., Chan M. Y., Thuya W. L., Lau J. Y., Wan S. C., Wong A. L., Zee Y. K., Lim R., Lee S. C., Ho P. C., Lee H. S., Chan A., Ansher S., et al. (2013) Glucuronidation by UGT1A1 is the dominant pathway of the metabolic disposition of belinostat in liver cancer patients. PLoS ONE 8, e54522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Anne M., Sammartino D., Barginear M. F., and Budman D. (2013) Profile of panobinostat and its potential for treatment in solid tumors: an update. Onco. Targets. Ther. 6, 1613–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Castellino S., O'Mara M., Koch K., Borts D. J., Bowers G. D., and MacLauchlin C. (2012) Human metabolism of lapatinib, a dual kinase inhibitor: implications for hepatotoxicity. Drug Metab. Dispos. 40, 139–150 [DOI] [PubMed] [Google Scholar]
- 60. Regan S. L., Maggs J. L., Hammond T. G., Lambert C., and Williams D. P., and Park B. K. (2010) Acyl glucuronides: the good, the bad and the ugly. Biopharm. Drug Dispos. 31, 367–395 [DOI] [PubMed] [Google Scholar]
- 61. LoGuidice A., Wallace B. D., Bendel L., Redinbo M. R., and Boelsterli U. A. (2012) Pharmacologic targeting of bacterial β-glucuronidase alleviates nonsteroidal anti-inflammatory drug-induced enteropathy in mice. J. Pharmacol. Exp. Ther. 341, 447–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Saitta K. S., Zhang C., Lee K. K., Fujimoto K., Redinbo M. R., and Boelsterli U. A. (2014) Bacterial β-glucuronidase inhibition protects mice against enteropathy induced by indomethacin, ketoprofen or diclofenac: mode of action and pharmacokinetics. Xenobiotica 44, 28–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Somasundaram S., Rafi S., Hayllar J., Sigthorsson G., Jacob M., Price A. B., Macpherson A., Mahmod T., Scott D., Wrigglesworth J. M., and Bjarnason I. (1997) Mitochondrial damage: a possible mechanism of the “topical” phase of NSAID induced injury to the rat intestine. Gut 41, 344–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Boelsterli U. A., Redinbo M. R., and Saitta K. S. (2013) Multiple NSAID-induced hits injure the small intestine: underlying mechanisms and novel strategies. Toxicol. Sci. 131, 654–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Femia A. P., and Caderni G. (2008) Rodent models of colon carcinogenesis for the study of chemopreventive activity of natural products. Planta Med. 74, 1602–1607 [DOI] [PubMed] [Google Scholar]
- 66. Megaraj V., Ding X., Fang C., Kovalchuk N., Zhu Y., and Zhang Q. Y. (2014) Role of hepatic and intestinal P450 enzymes in the metabolic activation of the colon carcinogen azoxymethane in mice. Chem. Res. Toxicol. 27, 656–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Laqueur G. L., Matsumoto H., and Yamamoto R. S. (1981) Comparison of the carcinogenicity of methylazoxymethanol-β-d-glucosiduronic acid in conventional and germfree Sprague-Dawley rats. J. Natl. Cancer Inst. 67, 1053–1055 [PubMed] [Google Scholar]
- 68. Takada H., Hirooka T., Hiramatsu Y., and Yamamoto M. (1982) Effect of β-glucuronidase inhibitor on azoxymethane-induced colonic carcinogenesis in rats. Cancer Res. 42, 331–334 [PubMed] [Google Scholar]
- 69. Gu D., McNaughton L., Lemaster D., Lake B. G., Gooderham N. J., Kadlubar F. F., Turesky R. J. (2010) A comprehensive approach to the profiling of cooked meat carcinogens 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, and their metabolites in human urine. Chem. Res. Toxicol. 23, 788–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zheng Z., Fang J. L., and Lazarus P. (2002) Glucuronidation: an important mechanism for detoxification of benzo[a]pyrene metabolites in aerodigestive tract tissues. Drug Metab. Dispos. 30, 397–403 [DOI] [PubMed] [Google Scholar]
- 71. Schmitt G., Aderjan R., Keller T., and Wu M. (1995) Ethyl glucuronide: an unusual ethanol metabolite in humans. Synthesis, analytical data, and determination in serum and urine. J. Anal. Toxicol. 19, 91–94 [DOI] [PubMed] [Google Scholar]
- 72. Palmer R. B. (2009) A review of the use of ethyl glucuronide as a marker for ethanol consumption in forensic and clinical medicine. Semin. Diagn. Pathol. 26, 18–27 [DOI] [PubMed] [Google Scholar]
- 73. Baranowski S., Serr A., Thierauf A., Weinmann W., Grosse Perdekamp M., Wurst F. M., and Halter C. C. (2008) In vitro study of bacterial degradation of ethyl glucuronide and ethyl sulphate. Int. J. Legal Med. 122, 389–393 [DOI] [PubMed] [Google Scholar]
- 74. Morini L., Politi L., Groppi A., Stramesi C., and Polettini A. (2006) Determination of ethyl glucuronide in hair samples by liquid chromatography/electrospray tandem mass spectrometry. J. Mass Spectrom. 41, 34–42 [DOI] [PubMed] [Google Scholar]
- 75. Chen G., Giambrone N. E. Jr., Dluzen D. F., Muscat J. E., Berg A., Gallagher C. J., and Lazarus P. (2010) Glucuronidation genotypes and nicotine metabolic phenotypes: importance of functional UGT2B10 and UGT2B17 polymorphisms. Cancer Res. 70, 7543–7552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Byrd G. D., Chang K. M., Greene J. M., and deBethizy J. D. (1992) Evidence for urinary excretion of glucuronide conjugates of nicotine, cotinine, and trans-3′-hydroxycotinine in smokers. Drug Metab. Dispos. 20, 192–197 [PubMed] [Google Scholar]
- 77. Montenegro M. A., Boiero M. L., Valle L., and Borsarelli C. D. (2012) Gum Arabic: more than an edible emulsifier. Products and Applications of Biopolymers. InTech. 10.5772/33783 [DOI] [Google Scholar]
- 78. Babiker R., Merghani T. H., Elmusharaf K., Badi R. M., Lang F., and Saeed A. M. (2012) Effects of Gum Arabic ingestion on body mass index and body fat percentage in healthy adult females: two-arm randomized, placebo controlled, double-blind trial. Nutr. J. 11, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chakdar H., Kumar M., Pandiyan K., Singh A., Nanjappan K., Kashyap P. L., and Srivastava A. K. (2016) Bacterial xylanases: biology to biotechnology. 3 Biotech. 6, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Goldin B. R., and Gorbach S. L. (1984) The effect of milk and Lactobacillus feeding on human intestinal bacterial enzyme activity. Am. J. Clin. Nutr. 39, 756–761 [DOI] [PubMed] [Google Scholar]
- 81. Beaud D., Tailliez P., and Anba-Mondoloni J. (2005) Genetic characterization of the β-glucuronidase enzyme from a human intestinal bacterium, Ruminococcus gnavus. Microbiology 151, 2323–2330 [DOI] [PubMed] [Google Scholar]
- 82. Dabek M., McCrae S. I., Stevens V. J., Duncan S. H., and Louis P. (2008) Distribution of β-glucosidase and β-glucuronidase activity and of β-glucuronidase gene gus in human colonic bacteria. FEMS Microbiol. Ecol. 66, 487–495 [DOI] [PubMed] [Google Scholar]
- 83. Gadelle D., Raibaud P., and Sacquet E. (1985) β-Glucuronidase activities of intestinal bacteria determined both in vitro and in vivo in gnotobiotic rats. Appl. Environ. Microbiol. 49, 682–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gloux K., Berteau O., El Oumami H., Béguet F., Leclerc M., and Doré J. (2011) A metagenomic β-glucuronidase uncovers a core adaptive function of the human intestinal microbiome. Proc. Natl. Acad. Sci. U.S.A. 108, 4539–4546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kim D. H., Hong S. W., Kim B. T., Bae E. A., Park H. Y., and Han M. J. (2000) Biotransformation of glycyrrhizin by human intestinal bacteria and its relation to biological activities. Arch. Pharm. Res. 23, 172–177 [DOI] [PubMed] [Google Scholar]
- 86. McBain A. J., and Macfarlane G. T. (1998) Ecological and physiological studies on large intestinal bacteria in relation to production of hydrolytic and reductive enzymes involved in formation of genotoxic metabolites. J. Med. Microbiol. 47, 407–416 [DOI] [PubMed] [Google Scholar]
- 87. Nakamura J., Kubota Y., Miyaoka M., Saitoh T., Mizuno F., and Benno Y. (2002) Comparison of four microbial enzymes in Clostridia and Bacteroides isolated from human feces. Microbiol. Immunol. 46, 487–490 [DOI] [PubMed] [Google Scholar]
- 88. Russell W. M., and Klaenhammer T. R. (2001) Identification and cloning of gusA, encoding a new β-glucuronidase from Lactobacillus gasseri ADH. Appl. Environ. Microbiol. 67, 1253–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Trottier J., Verreault M., Grepper S., Monté D., Bélanger J., Kaeding J., Caron P., Inaba T. T., and Barbier O. (2006) Human UDP-glucuronosyltransferase (UGT)1A3 enzyme conjugates chenodeoxycholic acid in the liver. Hepatology 44, 1158–1170 [DOI] [PubMed] [Google Scholar]
- 90. Theocharis A. D. (2002) Human colon adenocarcinoma is associated with specific post-translational modifications of versican and decorin. Biochim. Biophys. Acta 1588, 165–172 [DOI] [PubMed] [Google Scholar]
- 91. Halter C. C., Dresen S., Auwaerter V., Wurst F. M., and Weinmann W. (2008) Kinetics in serum and urinary excretion of ethyl sulfate and ethyl glucuronide after medium dose ethanol intake. Int. J. Legal Med. 122, 123–128 [DOI] [PubMed] [Google Scholar]