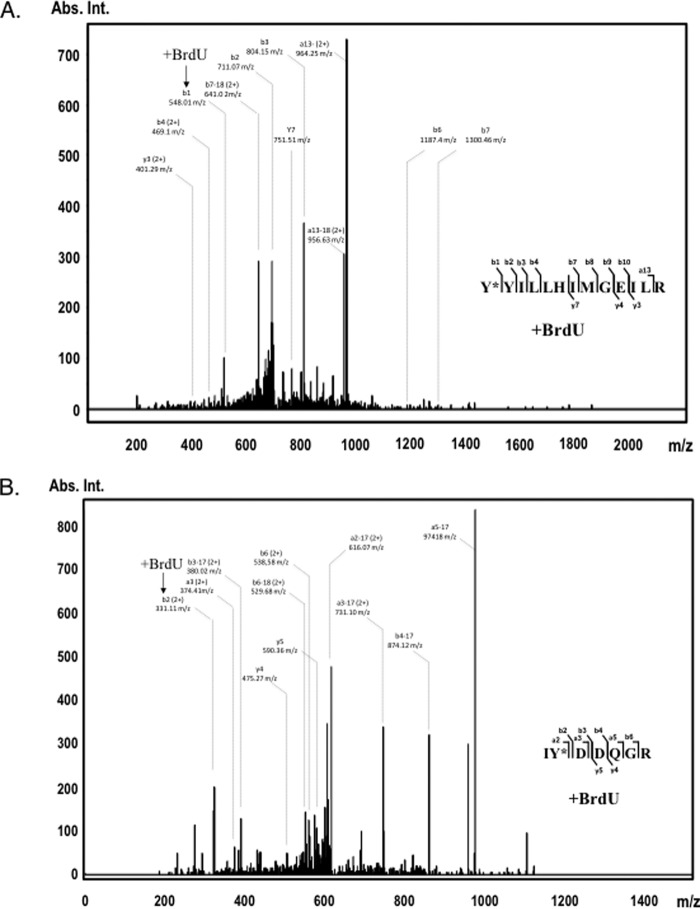
Figure 2.
Identification of A3G tyrosines 181 (top panel) and 315 (bottom panel) as cross-linked residues to 25-nt BrdU-modified ssDNA. UV light-induced cross-linking was performed with assembled A3G-ssDNA complex, and the cross-linked product was separated onto SDS-polyacrylamide gel and in-gel digested with trypsin. A3G tryptic peptides were analyzed on Q Exactive Plus Hybrid Quadrupole-Orbitrap mass spectrometer and searched with Mascot for amino acid residue BrdU modifications. Shown are A3G peptide MS/MS fragmentation spectra: aa 181–194 with BrdU-modified Tyr-181 (A) and aa 314–320 with BrdU-modified Tyr-315 (B). Peptide sequences are shown on the right. Most abundant b and y ion values and their m/z charge are marked on the spectra (no charge is shown for +1 fragment values). The positions of the BrdU-cross-linked fragments on the spectra are indicated with arrows. The complete list of identified b and y ions is presented in supplemental Tables 1 and 2 (1+ (monoisotopic) and 2+ mass/charge values). Abs. Int., absorbance intensity.