Figure 3.
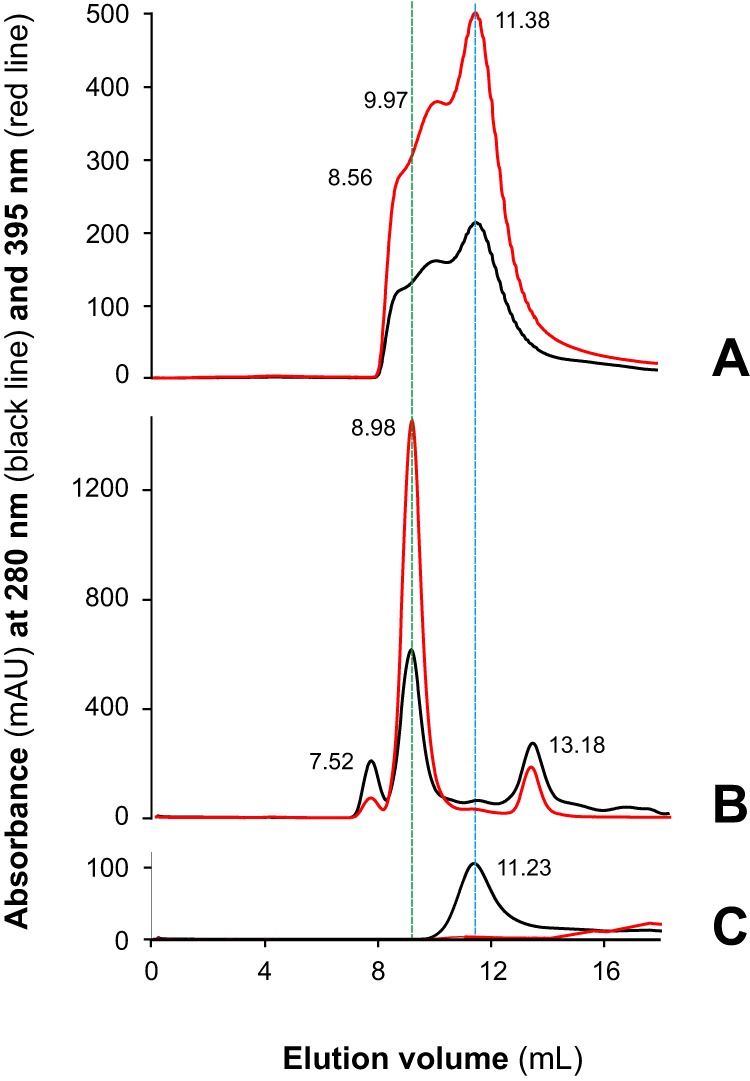
The sMF6p/FhHDM-1 protein tends to oligomerize and form high-molecular-mass complexes with hemin. Shown are elution profiles obtained by SEC (Superdex 75 HR 10/30) of sMF6p/FhHDM-1 (0.2-ml sample at 0.12 mm in PBS) applied to a column to which hemin (0.2-ml sample at 0.06 mm in PBS) was previously adsorbed (A), F. hepatica SAs diluted in PBS (B), and a sample of sMF6p/FhHDM-1 applied to a clean column in the absence of hemin (C). Absorbance at wavelengths of 280 (black) and 395 nm (red) are shown for protein and hemin, respectively. The elution volume of the major peaks is indicated above each peak. Hemin trapped by the column beads in A was eluted with sMF6p/FhHDM-1, forming complexes of several molecular masses between that of nMF6p/FhHDM-1·heme complexes (elution volume, 8.98 ml; B), and sMF6p/FhHDM-1 (elution volume, 11.23 ml; C), which forms oligomers of 35–40 kDa. mAU, milliabsorbance units.
