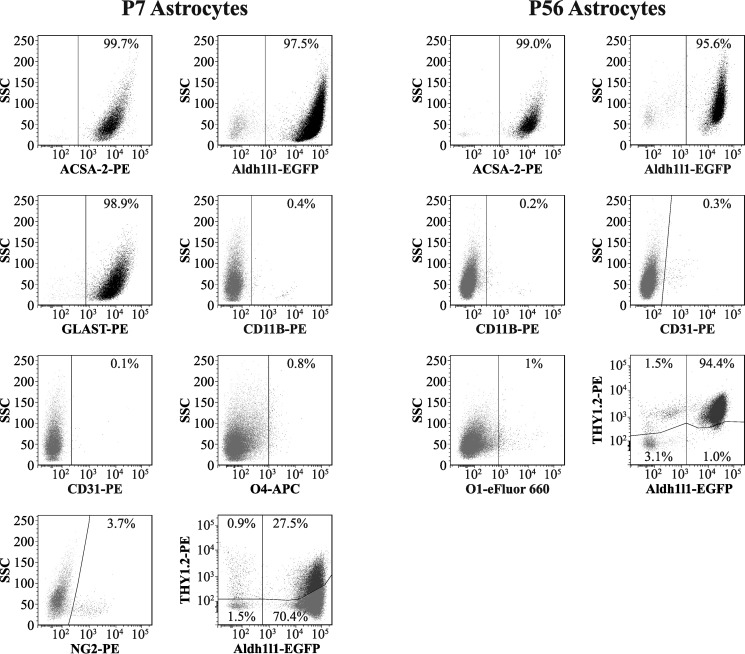
Figure 3.
Flow cytometry-based analysis of astrocytes isolated from young and adult mouse cortex. Live, non-fixed astrocytes were stained using a range of fluorescently conjugated antibodies for cell type-specific markers, including ACSA-2 and anti-GLAST (astrocytes), anti-CD11B (microglia), anti-O1 and anti-O4 (oligodendrocytes), anti-NG2 (oligodendrocyte precursors: NG2+ cells), anti-CD31 (endothelia), and anti-THY1.2 (neurons). (Full details of antibodies used can be found in Table 2.) We routinely see that > 95% of all cells purified are astrocytes, indicating that the preparation is of exceptional purity (consistent with data from qPCR analysis; Fig. 2). Trace amounts of all other cell types were detected (< 2%) except in the case of NG2+ cells, where levels were higher (∼4%, although this figure could be a slight underestimation due to the sensitivity of the epitope to papain; see “Experimental procedures”). Note that THY1.2 staining was performed on astrocytes isolated from the Aldh1l1-EGFP mouse line (which expresses EGFP specifically in astrocytes), because THY1.2 was previously reported to stain both neurons and astrocytes. Graphs show results from one representative experiment. This experiment was repeated twice on separate days with identical results. In total, two biological replicates were analyzed for each condition, with at least 10,000 cells analyzed per sample (effectively 1 technical replicate per sample). Lines in each plot delineate gates; numbers represent the proportion of cells in each particular gate.