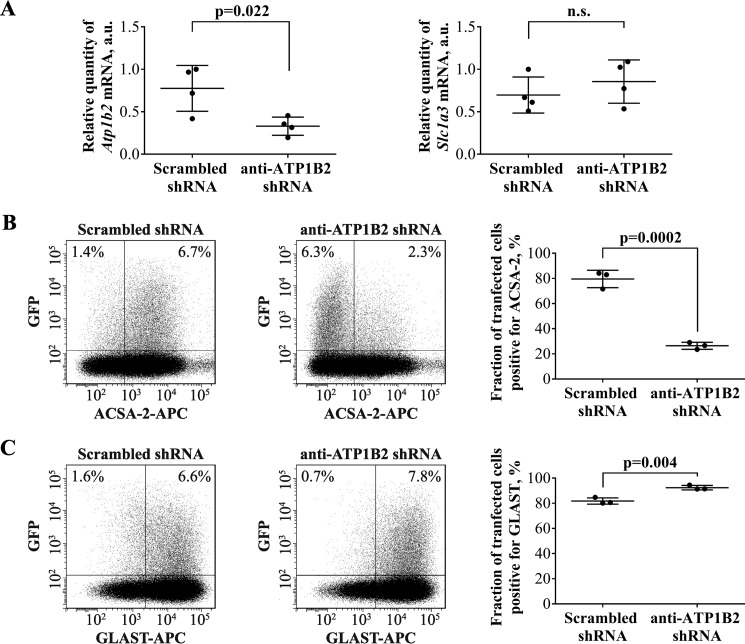
Figure 6.
ACSA-2 targets ATP1B2 on the plasma membrane of primary astrocytes. A, primary astrocytes expressing ATP1B2 shRNA for 7 days showed a 57% drop in the level of Atp1b2 mRNA, as judged by qPCR. This result was statistically significant (p = 0.022) when compared with a scrambled control shRNA. The level of an independent marker gene Slc1a3 (which encodes for the plasma membrane transporter GLAST) did not change upon expression of either shRNA. Results are from four sets of samples, prepared independently on four different days. Each sample was run using four technical replicates. a.u., arbitrary units. Graphs are plots of average ± S.D. (error bars). B, primary astrocytes expressing a scrambled control shRNA readily bind ACSA-2, as measured using flow cytometry (left). However, when primary astrocytes were transfected with shRNAs targeting Atp1b2, staining with ACSA-2 was markedly reduced (center). 7 days post-transfection, the average drop in the number of ACSA-2-positive cells for the most effective shRNA construct was 67% when compared with the scrambled control (quantified in the summary plot; right). This difference was statistically significant (p = 0.0002). C, astrocytes transfected with the same shRNAs as used in A (left, scrambled control; center, anti-Atp1b2) readily bind an antibody targeting the astrocyte plasma membrane protein GLAST, indicating the specificity of the shRNA for Atp1b2. Experiments were repeated three times with independent samples (effectively 1 technical replicate per sample) with similar results. Flow-cytometry plots show representative experiments. At least 100,000 cells were analyzed per sample. Graphs are plots of average ± S.D.