Figure 1.
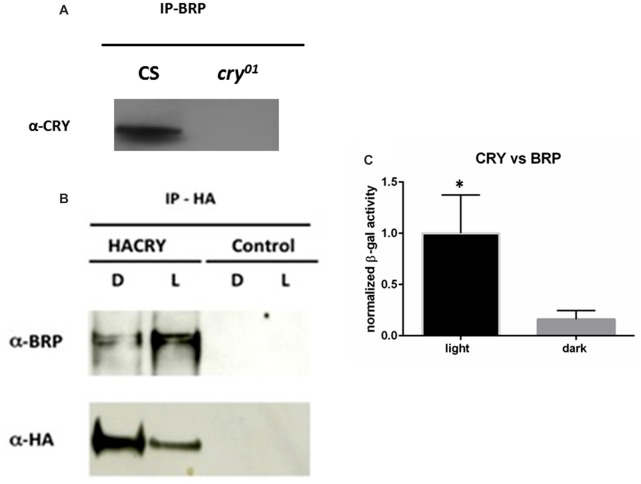
CRYPTOCHROME (CRY) interacts with the presynaptic protein BRUCHPILOT (BRP). (A) BRP was precipitated from whole head protein extracts of Canton S and cry01 flies with nc82 antibodies bound onto magnetic beads (Dynabeads). Co-immunoprecipitating (coIP) proteins were resolved by polyacrylamide gel electrophoresis (PAGE), transferred onto membrane by Western blot, and probed with α-CRY primary antibodies. This resulted in a band of ca. 60 kDa in the Canton S but not in the cry01 lane, suggesting a specific CRY-BRP interaction. (B) Samples were collected at ZT24 (darkness, D) and at ZT24 + 15 min of light (L) from tim > HACRY and yw;tim-GAL4 flies, the latter used as a negative control. Whole head protein samples were precipitated with a-HA antibodies and coIP BRP was revealed with nc82 antibodies specifically in flies overexpressing HACRY. BRP is visible as a double band suggesting that both the 170 and the 190 kDa isoforms coIP with CRY. Stronger bands in the L sample suggest that CRY and BRP form a complex more readily under light. (C) Full-length CRY (bait) was challenged with full-length BRP (prey) in a yeast 2 hybrid (Y2H) assay with β-galactosidase activity been a measure of interaction. As negative control, full-length CRY was challenged with empty prey vector, and the measured activity was considered as background. The graph reports relative β-galactosidase activity (Miller units) as mean ± SEM of seven independent clones analyzed in triplicate and corrected for background. The asterisk marks a statistically significant difference (t test, p < 0.0001) between the experiments conducted under darkness and under light.