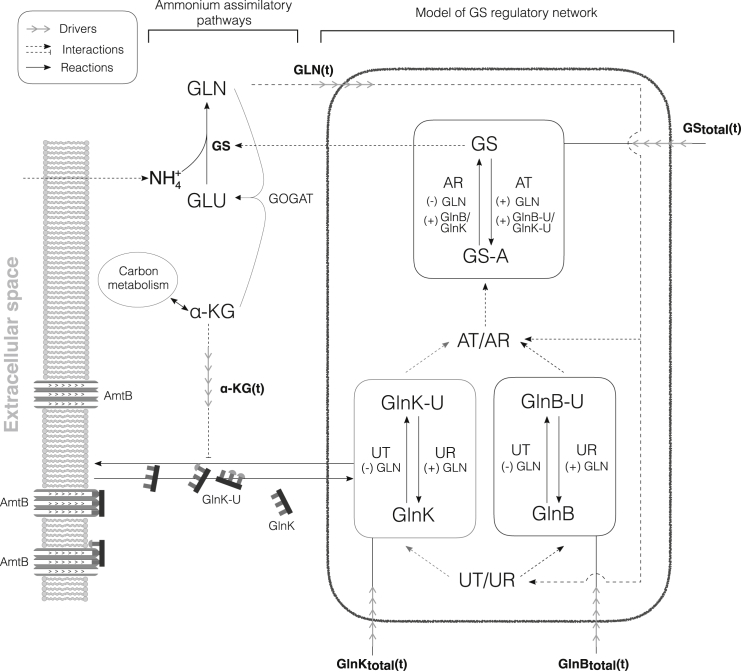
Figure 1.
Summary of the ammonium assimilatory system in E. coli. Under nitrogen-limited conditions, intracellular ammonium is assimilated almost exclusively through an enzymatic reaction catalyzed by GS. The activity of GS is controlled by the enzymes in the black box (which are the object of our model) as a function of the carbon (α-KG) and nitrogen (GLN) states of the cell. Our model describes the posttranslational states of GlnB, GlnK, and GS, which are modified by the enzymes AT/AR and UT/UR. The total concentrations of GlnB, GlnK, and GS, which reflect the transcriptional and translational responses of the cell, are measured experimentally and taken as drivers for the purposes of the model. Glutamine is sensed by UT/UR and transmitted via two complementary branches of signal transduction regulated by GlnB and GlnK. The focus of this study is GlnK. Trimers of GlnK form a complex with the membrane-bound transport protein AmtB, with the effect of blocking active ammonium import. During run-out and starvation, high levels of α-KG inhibit AmtB-GlnK complex formation, thus enabling active transport. Importantly, AmtB-GlnK complex formation sequesters GlnK, changing the amount accessible by UT/UR and AT/AR. The nitrogen and carbon information contained in the total and PTM levels of GlnB and GlnK are integrated with the level of GLN by the enzyme AT/AR to regulate the activity of GS.