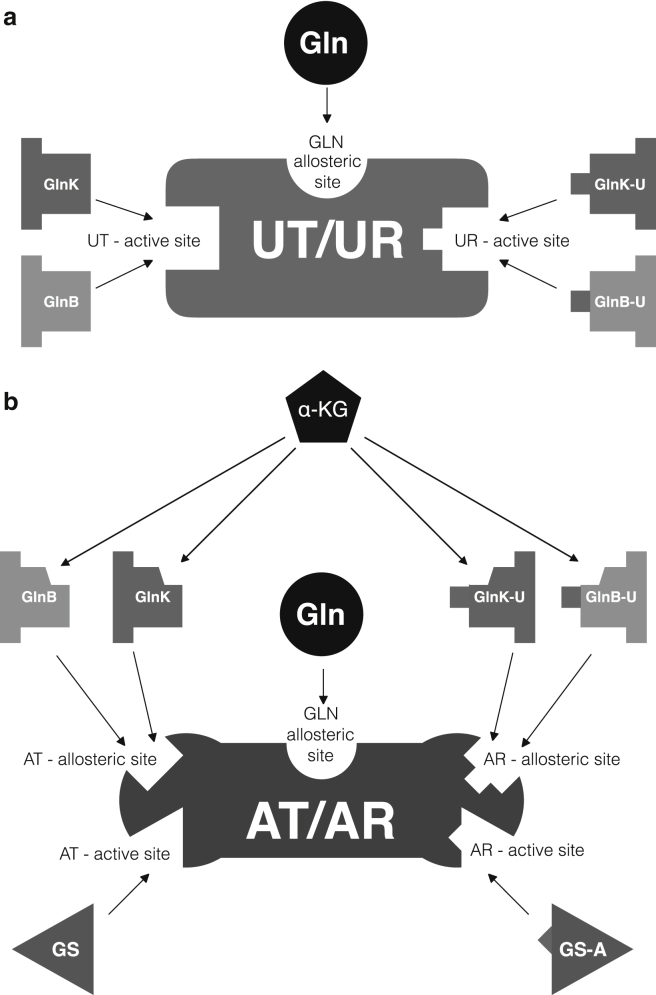
Figure 4.
Schematic illustration of the structure of UT/UR and AT/AR. (a) UT/UR has two distinct active sites: one binds GlnB-U and GlnK-U, whereas the other binds the unmodified GlnB and GlnK species. Hence GlnB and GlnK are competing substrates for UT/UR. In addition, UT/UR is allosterically regulated by glutamine, which activates the UR and inhibits the UT activity. (b) AT/AR has two separate active sites: one binds GS, the other GS-A. It also has three distinct allosteric sites: the first binds GlnB-U and GlnK-U and induces AR excitatory and AT inhibitory responses, the second binds GlnB and GlnK and induces AT excitatory and AR inhibitory responses, and the third binds GLN and modulates the rate of AT and AR responses. In addition, α-KG can bind to both the modified and unmodified GlnB/GlnK proteins, affecting their interaction with AT/AR.