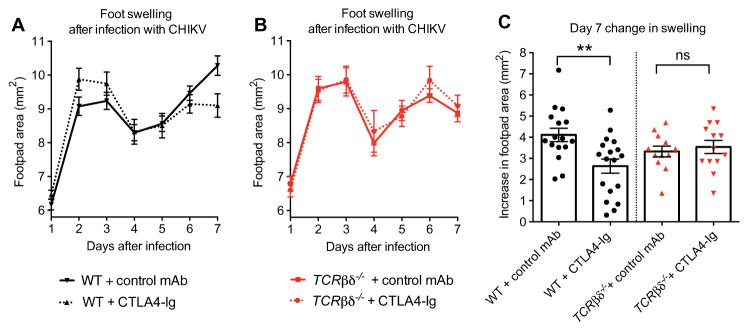
Fig. 7. Clinical assessment of the response to CTLA4-Ig treatment in CHIKV-infected WT and TCR.
βδ−/− mice.
Mice were inoculated with 103 FFU of CHIKV via a subcutaneous route. (A and B) Foot swelling in WT (A) and TCRβδ−/− (B) mice from day 1 through 7 in mice receiving at day 3 a single intraperitoneal injection of 300 μg of isotype control antibody or 300 μg of CTLA4-Ig. (C) Increase in foot swelling (area in square millimeter) on day 7 after infection in control- and CTLA4-Ig–treated WT and TCRβδ−/− mice. ns, not significant. Measurements were conducted in a blinded fashion. Results are pooled from two or three independent experiments with total n = 16 to 18 per group for WT animals and n = 12 to 13 per group for TCRβδ−/− animals. Data represent the means ± SEM. **P < 0.005 (two-way ANOVA with multiple comparisons for swelling curves; Mann-Whitney test for day 7 swelling).