Abstract
Background
Prostate cancer (PC) is a common noncutaneous malignancy in men. The incidence of PC is increasing at an alarming rate across the globe. Progression of PC is associated with elevated levels of interleukin-8 (IL-8) and cyclooxygenase-2 (COX-2) in malignant cells. Overexpression of these players is accompanied by chronic inflammation, increased angiogenesis, proliferation, migration, and inhibition of apoptosis. Moreover, their elevated circulating levels promote the disease progression from androgen-dependent to androgen-independent state. Thus, inhibiting the expression of IL-8 and COX-2 would be a promising target in the development of PC therapeutics. In this study, we investigated the inhibitory effects of Withania somnifera extract on highly metastatic, androgen-independent prostate cancer cell line (PC3). Additionally, we compared the real-time expression of IL-8 and COX-2 in prostate tissue samples.
Materials and methods
The cell viability and cytotoxicity of W. somnifera extract in PC3 cells was quantified colorimetrically by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide and lactate dehydrogenase leakage assay, respectively. Hematoxylin and eosin staining for histological examination, trypan blue, and acridine orange dyes to enumerate apoptotic and live cells, quantitative real-time polymerase chain reaction to determine the expression and flow cytometry to study the cell cycle analysis were used.
Results
We observed a significant decrease in the cell viability with a half-maximal inhibitory concentration (IC50) of 10 μg/mL. The expression levels of IL-8 and COX-2 in prostate tissue samples and in PC3 cells were predominantly high; however, the lowest dose of W. somnifera significantly inhibited the enhanced expression of IL-8 and COX-2 in PC3 cells in 24 hours. Furthermore, W. somnifera extract (10 μg/mL) irreversibly arrested the cell cycle in G2/M phase, which was evident from the rapid accumulation of PC3 cells significantly.
Conclusion
Our results indicate that inherent metastatic and selective inhibitory potential of W. somnifera against PC. W. somnifera may be a good therapeutic agent in addition to the existing drugs for PC. Further studies with more prostate tissue samples are warranted.
Keywords: Androgen-independent cells, Cyclooxygenase-2, Interleukin-8, Prostatic adenocarcinoma, Withania somnifera
1. Introduction
Prostate cancer is one of the leading causes of death among all cancers in elderly men worldwide.1, 2 Because of the growing and aging of the world’s population, by 2030 the prostate cancer burden is expected to reach 1.7 million cases.3 Progression of prostate cancer is orchestrated by various unusual physiological functions such as sustained cell proliferation, resisted cell death, increased cellular inflammation, excessive angiogenesis, and altered metastatic gene expression.4 Importantly, modulation of androgen receptor signals mediates the prostatic tumorigenesis.5 Surgery, hormonal therapy, radiotherapy, and chemotherapy are successful treatment strategies so far, but are limited based on patient’s age, hormonal deprivation, sensitivity, and resistance to therapeutic drugs.6, 7
In recent years, plant-derived extracts have drawn attention because of their richest bioactive compounds and their inherent ability to suppress the pathogenesis of cancers. Withania somnifera, one of the highly revered Indian herbs, possesses high medicinal value and has been traditionally used for the treatment of cancers, as well as inflammatory and neurological disorders.8, 9, 10, 11, 12, 13 Active principles of W. somnifera, which include alkaloids (anaferine), steroidal lactones (withanolides and withaferins), and saponins, are found to have anti-inflammatory activity in experimental animals.11, 14 Extensive studies are carried out using the W. somnifera crude extracts and demonstrated its inherent adaptogenicity, antibacterial, and immunomodulatory potential.15, 16, 17 It is effectively used in the prevention and treatment of stress-associated diseases such as arthritis, aging, arteriosclerosis, diabetes, hypertension, and malignancies.12, 18 W. somnifera showed cytotoxic effects in many cancer cell lines including prostate cancer cells. The ethanolic extract of W. somnifera suppressed the lipopolysaccharide-induced production of inflammatory cytokines in peripheral blood mononuclear cells.19 Suppression of angiogenesis and alteration of the cytoskeletal architecture have also been reported.14, 20
Accumulated research reports, epidemiological studies, and surveys highlight the beneficial and therapeutic effects of W. somnifera extracts; however, research studies addressing the influence of W. somnifera extracts in androgen-dependent and androgen-independent prostate cancer treatment are needed to better understand the mode of disease progression. It was reported that the levels of IL-8, a multifunctional chemokine, and COX-2, a proinflammatory and proangiogenic enzyme, are significantly high in prostatic adenocarcinoma; it may also play a key role in the proliferation, invasion, and metastasis in prostate cancer cells.21, 22 Hence, we investigated the effects of W. somnifera extract on proliferation, metastasis, and cell cycle transition in androgen-independent prostate cancer cells (PC3) through the expression of IL-8 and COX-2 in comparison with prostatic adenocarcinoma tissues. In addition, we examined the cell cycle checkpoint markers, cyclins A2, B1, and D1, using expression studies.
2. Materials and methods
2.1. Materials
Cryovials for tissue sample collection (Tarsons, Cat: 523040), reagents required for RNA isolation, namely, TRI reagent (Cat: T9424), Chloroform (Cat: V800116), isopropanol (Cat: V800228), formaldehyde (Cat: V800189), MOPS (Cat: M1254), DEPC (Cat: D5758) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) reagent (Cat: M2128), were purchased from Sigma-Aldrich. Mayer’s hematoxylin (Himedia Cat: S058) and eosin (Himedia Cat: S007) stains for histological staining, LDH Cytotoxicity Assay Kit (BioVision, Cat: K311-400), acridine orange (AO; Cat: A6014), propidium iodide (Cat: P4864), and all quantitative reverse transcription (qRT)-polymerase chain reaction (PCR) primers were purchased from Sigma-Aldrich. Fetal bovine serum (FBS; Gibco, Cat: 10270), Dulbecco’s modified Eagle’s medium (DMEM; Cat: SH30243.01), Antibiotic Antimycotic Solution (Cat: A002), Trypsin-EDTA (Himedia Labs, Cat: TCL007), mortar, pestle, and micropestle for grinding tissues were purchased from local vendors.
2.2. Prostate tissue collection and ethics
Prostate tissues samples (normal and prostatic adenocarcinoma) were collected from study participants in the Department of Urology, Velammal Medical College Hospital & Research Institute (VMCH & RI), Madurai, India, as per the Indian Council of Medical Research guidelines. The study was approved by the Institute Ethical Board of VMCH & RI for the identification, collection, and characterization of prostate samples. Both oral and written consent were obtained from the patients after a detailed briefing about the study. Trained surgical physician assistants at the hospital were involved in the sample collection procedure. Samples were then transported in frozen state using liquid nitrogen containers to the Department of Genetic Engineering, Madurai Kamaraj University, where the subsequent tissue processing were carried out. The study at Madurai Kamaraj University was approved by the Institutional Ethical Committee. Samples were authenticated and well characterized by physicians at the Department of Pathology, VMCH & RI, and grading of prostate tissues was done using Gleason scoring system.
2.3. Hematoxylin–eosin staining of prostate tissues
Prostate samples included in the study were obtained under the guidance of transrectal ultrasonography. Immediately after surgery, a portion of the prostate tissue was cryo-freezed using liquid nitrogen. The other portion was fixed in 10% neutral buffered formalin, processed, and embedded in a paraffin block. A 4-μm-thick section was taken from these blocks in microslides, and routine hematoxylin and eosin (H&E) staining was performed. Histopathological examination was carried out on samples from patients with prostate adenocarcinoma and patients with nonmalignant prostate lesions. In tumor cases, in addition to the histological type of the tumor, tumor grading (Gleason scoring system), status of perineural invasion, and in situ components were noted and documented in the report. Necessary images were also taken using a light microscope with an inbuilt camera (Coslab HL-23).
2.4. Collection and preparation of W. somnifera root extract
Roots of W. somnifera were collected from an ayurvedic medicinal farm (Madurai, Tamilnadu, India) identified by a botanist, and a voucher specimen was kept for future studies. The roots were rendered free from all impurities by washing with distilled water and shade-dried. Dried roots were grinded into powder and used (about 50 g) for each extraction with analytical-grade methanol (500 mL) in a Soxhlet apparatus as previously described.23 The extraction process was carried out for 24 hours, and the extract was concentrated using a rotary vacuum evaporator at 55°C. Concentrated crude extract was stored at –80°C. A portion of the concentrated extract was weighed and reconstituted in serum-free medium compatible for cell culture studies. The extract was filtered using 0.2-μm sterile syringe filters to avoid contamination.
2.5. Cell culture
The androgen-independent and highly metastatic human prostate cancer cell line (PC3) was purchased from the National Centre for Cell Sciences, Pune, India. As these cells were characterized as androgen receptor negative (ATCC No. HB8065), cells were grown in DMEM without any androgen supplements and were cultured with 10% FBS and 1× antibiotic antimycotic solution (containing penicillin, streptomycin, and amphotericin B). The cultures were maintained at 37°C, 5% CO2 and subcultured periodically using 0.25% trypsin–EDTA solution.
2.6. MTT assay
The MTT assay is a simple nonradioactive colorimetric assay used to directly measure cell proliferation or viability and indirectly cell toxicity. The MTT assay relies on the mitochondrial activity of cells, and thus serves as a biomarker of cell metabolism. To determine cell viability, the PC3 cells were seeded at a density of 1 × 104 cells/well in a 96-well plate at 37°C in 5% CO2. At 75–80% confluency, cells were treated with W. somnifera extract in varying concentrations and the well without W. somnifera extract acts as control. After 24 hours of treatment, media containing plant extracts were removed and 100 μL fresh medium was added along with 10 μL MTT dye solution (5 mg/mL in phosphate buffer pH 7.4). After 4 hours of incubation at 37°C in 5% CO2, the medium was removed and the formazan crystals were solubilized with 200 μL dimethyl sulfoxate. The absorbance of each well was read on a microplate reader at 575 nm. The relative cell viability (%) related to control wells containing cell culture medium without W. somnifera extract was calculated as [A]test/[A]control × 100.
2.7. Lactate dehydrogenase assay
Lactate dehydrogenase (LDH) is an oxidoreductase enzyme present in almost all organisms. Cells release LDH into the blood circulation (in vivo) or into culture supernatant (in vitro settings) when exposed themselves to any injurious stimuli or undergoing cell death. In this colorimetric LDH quantification assay, LDH reduces Nicotinamide Adenine Dinucleotide (Oxidized form) to Nicotinamide Adenine Dinucleotide-Hydrogen (Reduced form), which then interacts with a specific probe to produce a color that can be read at λmax = 450 nm.
2.8. Cell morphology assessment using phase contrast imaging
PC3 cells were seeded at 4 × 105 cells/well in six-well plates. Cells were treated with and without W. somnifera extracts at various concentrations for 24 hours at 37°C in 5% CO2. The control well contains cell culture medium without W. somnifera extract. At the end of 24 hours, the cells were rinsed thrice with phosphate-buffered saline (pH 7.4), and images were taken by inverted phase contrast microscope (TS 100; Nikon, Tokyo, Japan) with 10× magnification.
2.9. AO staining
To assess the occurrence of acidic vesicular organelles promoted by cytoplasmic proteins and lytic components, PC3 cells were treated with W. somnifera extracts for 24 hours and cells were stained with AO. Briefly, cells were incubated with AO (1 μg/mL) for 10 minutes then examined under a fluorescent microscope (Nikon, Japan) with 10× magnification.
2.10. Cell cycle analysis
Cells were seeded in a 35-mm plate at a density of 5 × 105 cells/plate, and cells were treated with 10 μg/mL of W. somnifera extract for 24 hours. Cells without treatment are taken as control. After 24 hours, cultured cells were collected in 1 mL extraction buffer (45mM Na2HPO4, 2.5mM citric acid, 0.1% Triton X-100, 1 mg/mL RNase, and 50 μg/mL propidium iodide, pH = 7.8), and analyzed using BD FACSAria III and the data were analyzed using Flow Jo software.
2.11. Quantitative real-time PCR studies
Quantitative RT-PCR was performed in an ABI 7500 sequence detection system (Applied Biosystems) using SyBr Green probe. Total RNA was isolated using TRI reagent. RT was performed with 1 μg DNAse treated RNA for 3 hours at 37°C. The expression levels of interleukin-8 (IL-8) and cyclooxygenase-2 (COX-2) were quantified against an internal control, β-actin. Relative transcript levels were determined according to the manufacturer’s protocol by calculating the 2ˆ(–ddcT) values.
2.12. Statistical analysis
We used Kolmogorov–Smirnov’s test to determine the normality of distribution. All clinical data were expressed as mean ± standard deviation. Comparisons between the two groups were performed using unpaired parametric Student t test or one-way analysis of variance, followed by Tukey’s multiple comparison post hoc test. Statistical significance was accepted if the null hypothesis was rejected at p ≤ 0.05. Data analyses were performed using Windows-based GraphPad PRISM (GraphPad Software, Inc., San Diego, CA, USA).
3. Results
3.1. Characteristics of study participants and samples
The study population comprised elderly male individuals with and without prostate cancer in the age group between 60 and 75 years. Prostate tissues were obtained from patients undergoing transurethral resection of the prostate for benign prostatic hyperplasia or transrectal biopsy for diagnosis of prostate cancer and transrectal ultrasonography channeling for metastatic prostate cancer at VMCH & RI. Based on the morphometric assessments (prostate size, weight) and urine flow pattern, individuals were sorted as patients with nonneoplastic [normal prostate (NP)] diseases and patients with prostate cancer (PC). In both groups, only those patients without tobacco smoking and alcohol drinking habits were considered for the study. Based on histological grading (Fig. 1) and Gleason scoring, PC individuals (prostatic adenocarcinoma) were identified, fixed for surgical procedures. A detailed questionnaire and an informed consent were obtained from all study participants prior to surgical procedures. Samples were collected with an utmost interest to confirm the expression levels of metastatic markers, and cell cycle associated “cyclins” are the same in both prostate tissues and PC cell line (PC3).
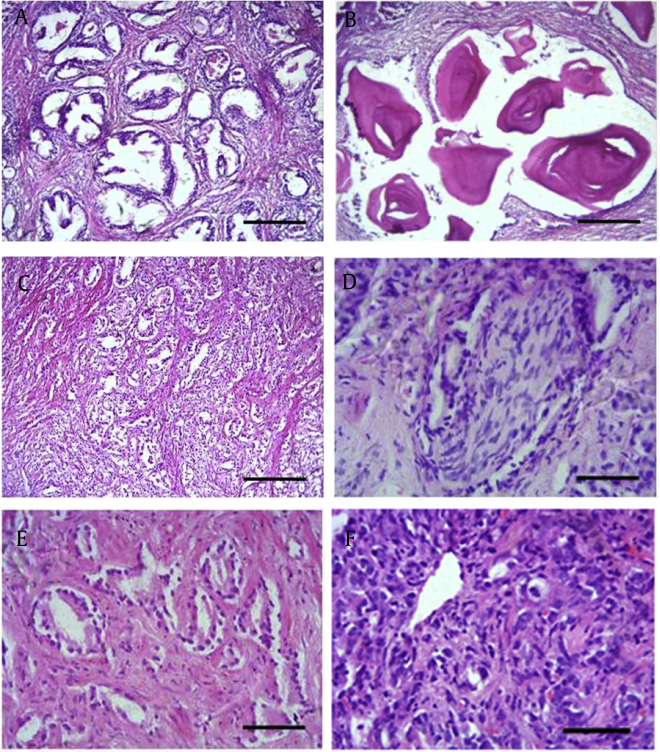
Fig. 1.
Histological examination and grading of prostate tissues. Representative light microscopic images showing hematoxylin and eosin staining of nonneoplastic and neoplastic lesions in study participants. Normal prostate has two unique features: (A) Presence of larger glands. (B) Presence of corpora amylacea, pink secretions-like structure. Two factors confirm prostate adenocarcinoma. (C) Absence of glands or smaller glands. (D) Presence of perineural invasion of nerve bundles. (E) Occurrence of neoplastic glandular structure in lower-grade tumors. (F) Absence of glands in higher-grade tumors.
3.2. Assessment of isolated total RNA
Total RNA was isolated from prostate tissue and PC3 cells (Fig. 2A, 2B) using TRI reagent. Integrity and purity of isolated RNA samples after DNase I treatment was quantified using nanodrop spectrophotometer and running 1% formaldehyde–agarose gel (Fig. 2C, 2D).
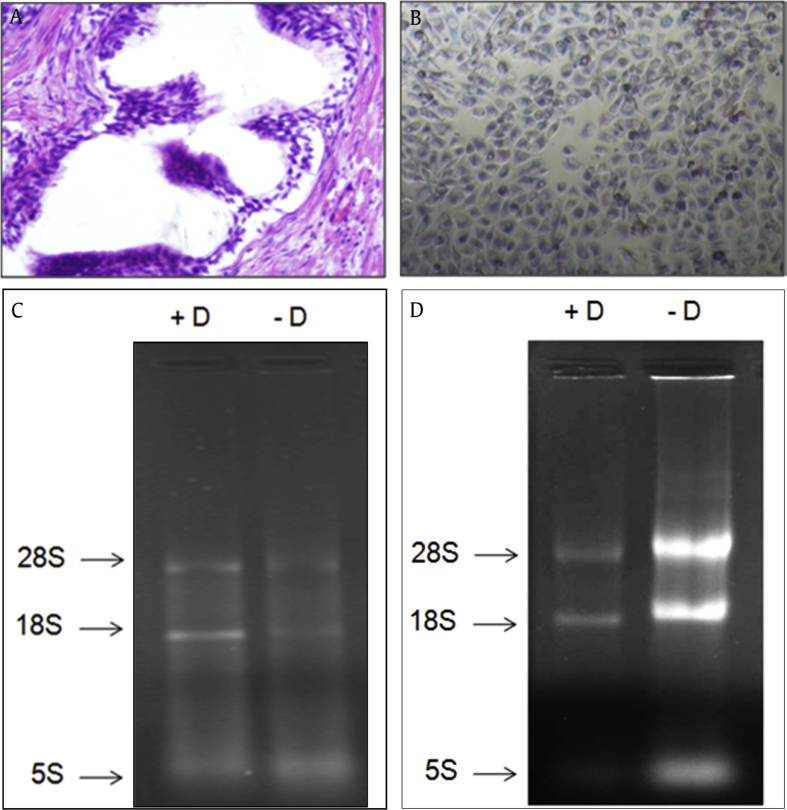
Fig. 2.
Assessment of total RNA. Representative images of prostate tissue sample and prostate cancer cell line (PC3), from which total RNA was isolated using TRI reagent. (A) Prostate tissue sample. (B) Prostate cancer cell line (PC3). 1% Formaldehyde–agarose gel showing the integrity and purity of isolated RNA with (+D) and without (–D) DNAse-I treatment. (C) Prostate tissue sample. (D) Prostate cancer cell line (PC3).
3.3. W. somnifera extract exhibits reduced viability and increased cytotoxicity in PC3 cells
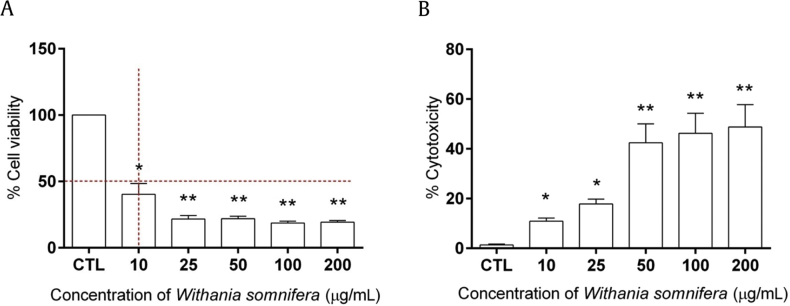
Cell viability and cytotoxicity of W. somnifera extract at different concentrations (10 μg/mL, 25 μg/mL, 50 μg/mL, 100 μg/mL, 200 μg/mL) was carried out in PC3 cell line at 24 hours, to determine the IC50 (50% growth inhibition) value using MTT assay. The percentage cell viability of PC3 cells significantly decreased (from 40% to 20%) with increasing concentrations of W. somnifera extract as shown (Fig. 3A). The half-minimal cytotoxicity (IC50 value) in PC3 cells was found at the 10 μg/mL concentration of W. somnifera with more than 40% decrease in viable cells. Staining of PC3 cells with trypan blue dye further indicated that W. somnifera extract induces uptake of trypan blue, suggesting cellular death (not shown). Interestingly, the percentage cytotoxicity of PC3 cells significantly increased (from 10% to 48%) with increasing concentrations (10 μg/mL, 25 μg/mL, 50 μg/mL, 100 μg/mL, 200 μg/mL) of W. somnifera extract quantified in cell culture supernatant using LDH cytotoxicity assay (Fig. 3B). Hence, it was evident that W. somnifera extract exhibits antiproliferative potential in androgen-independent prostate cancer cell line (PC3).
Fig. 3.
Withania somnifera decreases cell viability of prostate cancer cells. Cell viability and cytotoxicity of W. somnifera was evaluated using prostate cancer cell line (PC3). (A) Bar graph showing the % cell viability measured by MTT assay. (B) Bar graph showing the % cytotoxicity measured by LDH assay in PC3 cells treated with various concentrations of W. somnifera for 24 hours. Data are presented as mean ± SEM of three separate experiments. *P < 0.01; **P < 0.001 versus control. CTL, control; LDH, lactate dehydrogenase; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; SEM = standard error of the mean.
3.4. Assessment associated with cellular morphology in PC3 cells
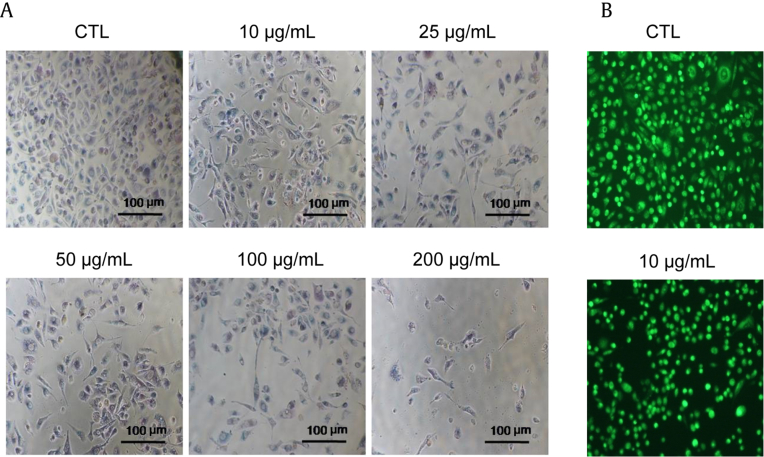
To check whether W. somnifera treatments induced alteration in the morphology of PC3 cells at various concentrations, we carried out imaging under a phase contrast microscope (Nikon Eclipse TS100) after 24 hours. The cell morphology of W. somnifera-treated cells was found to be irregular and stretched out. Furthermore, the nucleus was enlarged with fine fenestrations in the cell membrane. All images were taken in 10× magnification. As concentration of W. somnifera increases, confluency of cells decreases with shrinkages as shown (Fig. 4A). Staining of PC3 cells with AO dye further indicated that W. somnifera extract at 10 μg/mL is able to demonstrate that cells are not intact as seen in control cells without treatment (Fig. 4B).
Fig. 4.
Morphological assessment of Withania somnifera-treated PC3 cells at 24 hours. (A) Representative phase contrast images showing morphology of PC3 cell treated with various concentrations of W. somnifera, acridine orange staining. (B) Phase contrast images showing live cells after 24 hours. (Scale: 100 μm = 10× magnification.) CTL, control.
3.5. Cell cycle analysis
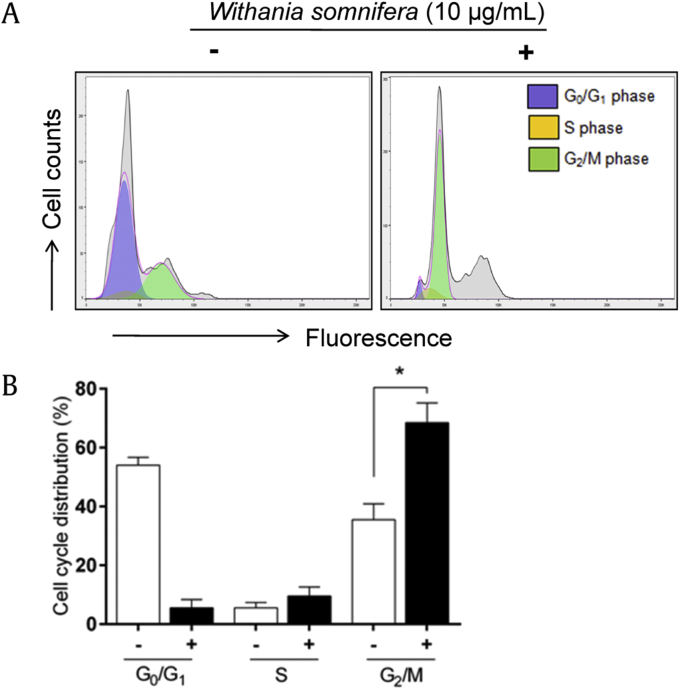
We analyzed the effect of W. somnifera extract on cell cycle progression. Overnight serum starvation was done to achieve 80% synchronization of cells in G0/G1 phase. PC3 cells were then treated with 10 μg/mL W. somnifera, and cells without treatment acting as control were analyzed 24 hours after treatment using propidium iodide labeling (Fig. 5A). The percentage of PC3 cells decreased from 63% to 3% in G0/G1 phase, increased from 3% to 6% in S phase, and significantly increased from 30% to 56% in G2/M phase (P = 0.044). The accumulation of cells was found to be greater in G2/M phase, suggesting a cell cycle arrest at G2/M phase. Such arrest at G2/M phase indicates inhibition of cell proliferation by the extract in PC3 cells (Fig. 5B).
Fig. 5.
Cell cycle analysis. (A) Representative flow cytogram showing PC3 cells in various phases of cell cycle with (10 μg/mL) and without Withania somnifera treatment. (B) Bar graph summarizing data, which are represented as mean ± standard deviation. *P < 0.05 versus control.
3.6. W. somnifera extract inhibits the expression of IL-8 and COX-2 in PC3 cells
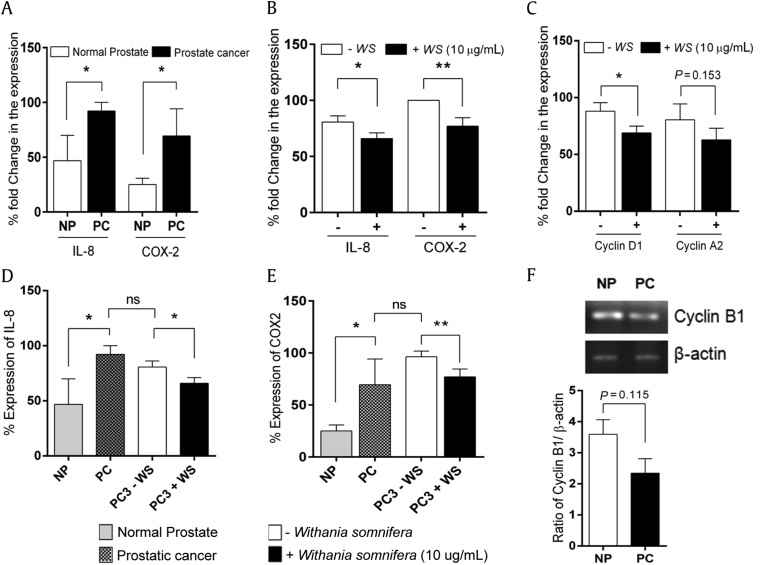
We quantified the real-time expression of metastatic markers, IL-8 and COX-2, in prostate cancer cells (PC3) with and without W. somnifera extract treatment in a 5% CO2 incubator for 24 hours. To also compare the expression pattern of these markers in tissues, we have processed in parallel the prostate tissue samples obtained from study participants (NP and PC, n = 3 in each group). The primers used in this study for qRT-PCR analysis are given in Table 1. We observed a significant increase in the percentage fold expression of IL-8 (P = 0.032) and COX-2 (P = 0.038) in prostatic adenocarcinoma (PC) tissues compared with the normal prostate (NP; Fig. 6A). Similarly, the expression levels of IL-8 and COX-2 were observed to be as high as 80% and 100%, respectively, in prostate cancer cells (PC3) without any W. somnifera extract treatment. After 24 hours of treatment with 10 μg/mL W. somnifera extract, PC3 cells showed a significant decrease in the expression levels of IL-8 (P = 0.028) and COX-2 (P = 0.005; Fig. 6B).
Table 1.
List of primers used for RT-PCR studies.
| Oligo names | Forward primers sequence (5′–3′) | Reverse primers sequence (5′–3′) |
|---|---|---|
| IL-8 | ATAAAGACATACTCCAAACCTTTCCAC | AAGCTTTACAATAATTTCTGTGTTGGC |
| COX-2 | CCCATGTCAAAACCGAGGTG | CCGGTGTTGAGCAGTTTTCTC |
| Cyclin D1 | TGAGAGAAAAAGGTCCTAGC | GTAGCAGCTACTGTAGACAG |
| Cyclin B1 | CAGATGTTTCCATTGGGCTT | TACCTATGCTGGTGCCAGTG |
| Cyclin A2 | CACTCACTGGCTTTTCATCTTCT | TGAAGATGCCCTGGCTTTTA |
| β-Actin | CCTTGCACATGCCGGAG | GCACAGAGCCTCGCCTT |
COX-2, cyclooxygenase-2; IL-8, interleukin-8; RT-PCR, reverse transcription-polymerase chain reaction.
Fig. 6.
Quantitative and semiquantitative reverse transcription-polymerase chain reaction (RT-PCR) analysis. Bar graphs showing the quantitative RT-PCR expression levels of interleukin-8 (IL-8) and cyclooxygenase-2 (COX-2). (A) Prostate tissue samples. (B) Prostate cancer cell line (PC3). (C) Bar graph showing fold change in cyclin D1 and A2 expression. Bar graphs showing the comparison in expression levels of IL-8 and COX-2. (D) Prostate tissue samples. (E) Prostate cancer cell line (PC3). (F) Ratio of cyclin B1 normalized with β-actin was carried out by semiquantitative RT-PCR. *P < 0.05, **P < 0.01 versus control. NP, normal prostate; ns, not significant; PC, prostate cancer; WS, Withania somnifera.
3.7. W somnifera extract attenuates the expression of cell cycle checkpoints in PC3 cells
We proceeded to determine the expression levels of the cell checkpoint markers, especially cyclins A2, B1, and D1, in both PC3 cells and in prostate tissue samples using quantitative RT-PCR. The expression of cyclin D1 (P = 0.026) was found to be significantly decreased, whereas cyclin A2 (P = 0.153) and B1 (P = 0.115) levels were found to be attenuated. We compared the expression pattern of cyclins A2 and D1 using qRT-PCR (Fig. 6C) and that of cyclin B1 by the semiquantitative RT-PCR method (Fig. 6F) using PC3 cells and prostate tissues, respectively, to better understand the inhibitory effects of W. somnifera extract over cell cycle regulation.
4. Discussion
Prostatic adenocarcinoma is a highly metastatic, nonandrogenic, noncutaneous malignancy in men.1, 24 At this stage, the disease is incurable and causes significant mortality. Currently available therapeutic and disease management strategies such as hormonal therapy, radiotherapy, chemotherapy, and clinical surgery are successful to some extent in the prevention and control of prostatic tumorigenesis and metastasis.25 Determination of appropriate treatment regime is purely based on patient’s age, disease status, compatibility to chemotherapeutics, and tolerance to androgen deprivation. Therefore, patients are in need of alternative and efficient treatment strategies that can combat the motility of cancer cells, thereby preventing the disease progression and prolonging the survival rate.
Epidemiological studies pertaining to the therapeutics of dietary agents have shown that consumption of naturally available fruits, vegetables, and spices lowers the incidence of cancers.26 Dietary agents are found to possess many biologically active compounds that are ubiquitous in plants. W. somnifera, an Indian ayurvedic medicinal plant, has been traditionally used for the treatment of cancers, as well as inflammatory and neurological disorders.8, 9, 10, 11, 12, 13 In the present study, we investigated the effect of W. somnifera root extract on proliferation, metastasis, and cell cycle transition in androgen-independent prostate cancer cells (PC3) through the expression of IL-8 and COX-2 in comparison with prostatic adenocarcinoma tissues.
To address the first and foremost question—whether the isolated prostate derived tissues and the prostate cancer cells have similar malignant features—we carried out a detailed microscopic and histological evaluation. Prostate tissues and prostate cells were well characterized based on H&E staining and AO staining, respectively. Consequently, we assessed the proliferative potential of PC3 cells in in vitro androgen-deprived culture condition in response to the administration of W. somnifera extract at 24 hours. It was evident that cells without treatment grow to a maximum extent with no compromise in viability, whereas cells with varying concentrations of W. somnifera grow with reduced viability. This is further confirmed by the trypan blue exclusion assay, where viable (intact) cells exclude the uptake of dye in contrast to the nonviable cells. Further calorimetric analysis of the cell culture supernatants (spent medium) reveals that W. somnifera extract possess cytotoxic properties, which were quantified by the levels of LDH released by PC3 cells. Cells without any treatment do not show the release of LDH, whereas cells treated with various concentrations of W. somnifera released significant amounts of LDH—thus confirming that W. somnifera extract exhibits antiproliferative potential in androgen-independent prostate cancer cells.
IL-8 and COX-2 were considered to be the key player in the regulation of metastasis and angiogenesis in prostate cancer.21, 22 Inflamed neoplastic cells secrete excess IL-8, which enhances the growth and tumorigenicity in human prostate cancer mediated by the induction of MMP-9 expression.27, 28, 29, 30, 31, 32, 33, 34 When compared with normal tissues, malignant tissues were found to induce COX-2 expression constitutively; this remains throughout the process from dysplasia to metastasis. This adaptation further favors tumor progression, contributes in cellular adhesion to the extracellular matrix, and makes cells resistant to apoptosis.22 In the current study, we examined the expression of IL-8 and COX-2 levels in both prostate tissues and in PC3 cells by qRT-PCR analysis. Our results, both in prostate tissues and in PC3 cell lines, showed an increased expression of IL-8 and COX-2. Interestingly, the increased expression of IL-8 and COX-2 in PC3 cells were significantly attenuated by the lowest dose of W. somnifera treatment in 24 hours. This indicates that the influential role played by IL-8 and COX-2 in prostate cancer progression can be blocked by the administration of W. somnifera root extract, thus confirming that W. somnifera extract exhibits antimetastatic and antiangiogenic potential by attenuating the expression of these major players and thereby inhibiting the progression of prostate cancer. Moreover, cell cycle analysis reveals that minimum of cells were in the G0/G1 and S phases, but maximum of cells accumulated in the G2/M phase in response to W. somnifera treatment, indicating the inherent ability of W. somnifera in blocking the cell cycle progression by suppressing the endogenous IL-8 or its receptors attenuating cyclin D1.25, 35
Based on our results, we believed that W. somnifera-induced selective inhibitory potential against androgen-independent prostate cancer was achieved by attenuating proliferative, metastatic and angiogenic signals mediated by IL-8 and COX-2 expression and by arresting cell cycle in G2/M phase (Fig. 7). These results were reliable and consistent with previous reports. Seaton et al30 have shown that IL-8 signals promote the androgen-independent proliferation of prostate cancer cells by inducing the expression and activation of androgen receptors. Richardsen et al33 have shown that in cancerous prostatic tissues due to insensitivity and adaptability to the apoptotic signals, the expression of COX-2 was enhanced.33 In general, activation of cell cycle checkpoints play a major role in maintaining genomic stability. Consequently, W. somnifera blocks the cell cycle transition in G2/M phase and thereby prevents the entry of cells into mitotic phase and leads to DNA damage and cell death. It is known that the key player involved in G2 to M phase transition is cyclin B1. Notably, W. somnifera downregulates the expression of cyclins A2, B1, and D1. Moreover, it should be noted that the expression of p53 is crucial for cell death in cancerous tissues; however, PC3 cells do not express p53.36 Hence, our results indicate that W. somnifera induces irreversible G2/M arrest in a p53-independent manner, which was associated with the initiation of mitotic catastrophe (delayed mitosis linked cell death) in androgen-independent prostate cancerous cells.37, 38 This study opens up new avenues and lays a platform for future clinical investigation and therapeutic modalities for the prevention and treatment of prostate cancer. In conclusion, our results suggest that regular intake of W. somnifera (a good dietary agent) may prolong life expectancy in prostatic adenocarcinoma patients along with the prescribed drug of choice.
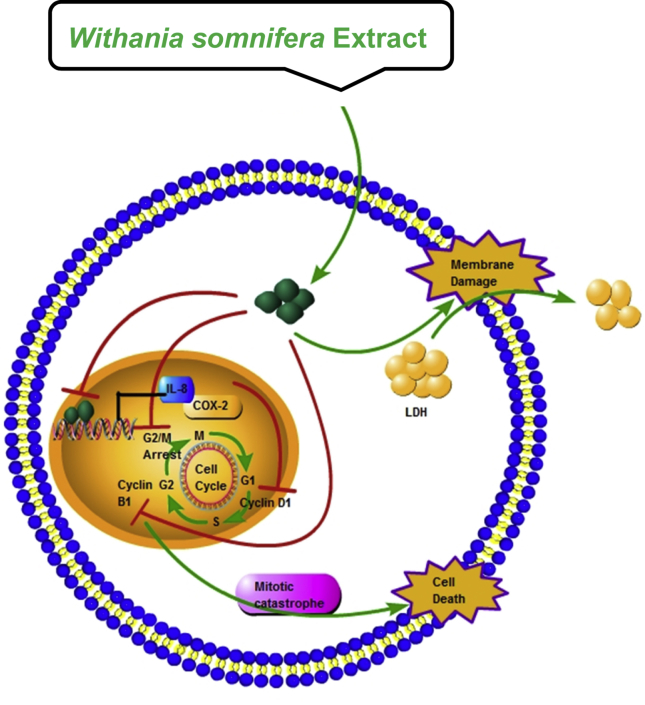
Fig. 7.
Schematic diagram showing the overall action of Withania somnifera extract in prostate cancer cell line (PC3) cells. Root extract was prepared from the roots of W. somnifera and administered to PC3, which is androgen-independent and p53-independent. W. somnifera extract (10 μg/mL) efficiently inhibits the expression of interleukin-8 (IL-8) and cyclooxygenase-2 (COX-2), arrest cell cycle at G2/M phase and initiates cell death via mitotic catastrophe process (delayed mitosis). This also releases lactate dehydrogenase upon cell membrane damage.
4.1. Limitations
There are several limitations in the current study. Because of the small sample size, we could not perform correlation analysis between the observed Gleason scores with the hematological parameters. Certain clinical observations and malignant features did not attain statistically significant level. The prostate tissues collected in the study were used to compare the expression status of the key markers in prostate cancer cell line (PC3) only at the mRNA level.
Conflicts of interest
There is no conflicts of interest.
Acknowledgments
The authors acknowledge the dean of Velammal Medical College Hospital & Research Institute (VMCH & RI), Madurai, for the approval and coordination in the sample collection; they also thank the physicians and laboratory assistants of the Department of Urology and Pathology for technical assistance and support in sampling and in histological studies. The authors likewise acknowledge the MKU/SBS/DBT–IPLS program for granting them permission to use the FACS facility. This study was partly supported by Department of Biotechnology – RGYI scheme (BT-R13179/GBD/27/213/2009), Government of India, and Madurai Kamaraj University — Internal funding.
References
- 1.Muhammad N.B. Epidemiology of prostate cancer. Asian Pac J Cancer Prev. 2015;16:5137–5141. doi: 10.7314/apjcp.2015.16.13.5137. [DOI] [PubMed] [Google Scholar]
- 2.Jophy V., Priya M.K., Nisarg M. Asian Pac J Cancer Prev. 2016;17:2255–2258. [Google Scholar]
- 3.Ferlay J., Shin H.R., Bray F. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Datta D., Aftabuddin M., Gupta D.K., Raha S., Sen P. Human prostate cancer hallmarks map. Sci Rep. 2016;1:30691. doi: 10.1038/srep30691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris W.P., Mostaghel E.A., Nelson P.S., Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6:76–85. doi: 10.1038/ncpuro1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nacusi L.P., Tindall D.J. Targeting 5 alpha-reductase for prostate cancer prevention and treatment. Nat Rev Urol. 2011;8:378–384. doi: 10.1038/nrurol.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh N., Verma P., Pandey B.R., Gilca M. Role of Withania somnifera in prevention and treatment of cancer: an overview. Int J Pharm Sci Drug Res. 2011;3:274–279. [Google Scholar]
- 9.Avani R.V., Shivendra V.S. Molecular targets and mechanisms of cancer prevention and treatment by withaferin A, a naturally occurring steroidal lactone. AAPS J. 2014;16:1–10. doi: 10.1208/s12248-013-9531-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirjalili M.H., Moyano E., Bonfill M., Cusido R.M., Palazón J. Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules. 2009;14:2373–2393. doi: 10.3390/molecules14072373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattacharya S.K., Satyan K.S., Ghosal S. Antioxidant activity of glycowithanolides from Withania somnifera. Indian J Exp Biol. 1997;35:236–239. [PubMed] [Google Scholar]
- 12.Mishra L.C., Singh B.B., Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (Ashwagandha): a review. Altern Med Rev. 2000;5:334–346. [PubMed] [Google Scholar]
- 13.Muskan G., Gurcharan K. Aqueous extract from the Withania somnifera leavesas a potential antineuroinflammatory agent: a mechanistic study. J Neuroinflamm. 2016;13:193. doi: 10.1186/s12974-016-0650-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohan R., Hammers H.J., Bargagna-Mohan P., Zhan X.H., Herbstritt C.J., Ruiz A. Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis. 2004;7:115–122. doi: 10.1007/s10456-004-1026-3. [DOI] [PubMed] [Google Scholar]
- 15.Ghosal S., Lal J., Srivastava R.S., Bhattacharya S.K., Upadhyay S.N., Jaiswal A.K. Immunomodulatory and CNS effects of sitoindosides IX and X, two new glycowithanolides from Withania somnifera. Phytother Res. 1989;2:201–206. [Google Scholar]
- 16.Owais M., Sharad K.S., Shehbaz A., Saleemuddin M. Antibacterial efficacy of Withania somnifera (ashwagandha) an indigenous medicinal plant against experimental murine salmonellosis. Phytomedicine. 2005;12:229–235. doi: 10.1016/j.phymed.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Rasool M., Varalakshmi P. Immunomodulatory role of Withania somnifera root powder on experimental induced inflammation: an in vivo and in vitro study. Vascul Pharmacol. 2006;44:406–410. doi: 10.1016/j.vph.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Singh N. Institute of Nuclear Medicine and Allied Sciences (DRDO, Govt. of India); 2006. Ayurvedo-herbal medicines – the need of the time – herbal drugs, a twenty-first century perspective; pp. 535–547. [Google Scholar]
- 19.Singh D., Aggarwal A., Maura R., Naik S. Withania somnifera inhibits NF-κB and AP-1 transcription factors in human peripheral blood and synovial fluid mononuclear cells. Phytother Res. 2007;21:905–913. doi: 10.1002/ptr.2180. [DOI] [PubMed] [Google Scholar]
- 20.Falsey R.R., Marron M.T., Gunaherath G.M., Shirahatti N., Mahadevan D., Gunatilaka A.A. Actin microfilament aggregation induced by withaferin A is mediated by annexin II. Nat Chem Biol. 2006;1:33–38. doi: 10.1038/nchembio755. [DOI] [PubMed] [Google Scholar]
- 21.Singh R.K., Lokeshwar B.L. Depletion of intrinsic expression of interleukin-8 in prostate cancer cells causes cell cycle arrest, spontaneous apoptosis and increases the efficiency of chemotherapeutic drugs. Mol Cancer. 2009;8:57. doi: 10.1186/1476-4598-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aparicio Gallego G., Díaz Prado S., Jiménez Fonseca P., Garcia Campelo R., Cassinello Espinosa J., Anton Aparicio L.M. Cyclooxygenase-2 (COX-2): a molecular target in prostate cancer. Clin Transl Oncol. 2007;9:694–702. doi: 10.1007/s12094-007-0126-0. [DOI] [PubMed] [Google Scholar]
- 23.Lavie D., Kirson I., Glotter E. Constituents of Withania somnifera Dun: Part X. The structure of withanolide D. Isr J Chem. 1968;6:671–678. [Google Scholar]
- 24.Tai S., Sun Y., Squires J.M., Zhang H., Oh W.K., Liang C.Z. PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate. 2011;71:1668–1679. doi: 10.1002/pros.21383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy R.V., Suman S., Das T.P., Luevano J.E., Damodaran C. Withaferin A, a steroidal lactone from Withania somnifera, induces mitotic catastrophe and growth arrest in prostate cancer cells. J Nat Prod. 2013;76:1909–1915. doi: 10.1021/np400441f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aggarwal B.B., Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Araki S., Omori Y., Lyn D., Singh R.K., Meinbach D.M., Sandman Y. Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Res. 2007;67:6854–6862. doi: 10.1158/0008-5472.CAN-07-1162. [DOI] [PubMed] [Google Scholar]
- 28.Inoue K., Slaton J.W., Eve B.Y., Kim S.J., Perrotte P., Balbay M.D. Interleukin 8 expression regulates tumorigenicity and metastases in androgen-independent prostate cancer. Clin Cancer Res. 2000;6:2104–2119. [PubMed] [Google Scholar]
- 29.Kim S.J., Uehara H., Karashima T., Mccarty M., Shih N., Fidler I.J. Expression of interleukin-8 correlates with angiogenesis, tumorigenicity, and metastasis of human prostate cancer cells implanted orthotopically in nude mice. Neoplasia. 2001;3:33–42. doi: 10.1038/sj.neo.7900124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seaton A., Scullin P., Maxwell P.J., Wilson C., Pettigrew J., Gallagher R. Interleukin-8 signaling promotes androgen-independent proliferation of prostate cancer cells via induction of androgen receptor expression and activation. Carcinogenesis. 2008;29:1148–1156. doi: 10.1093/carcin/bgn109. [DOI] [PubMed] [Google Scholar]
- 31.Kooijman R., Himpe E., Potikanond S., Coppens A. Regulation of interleukin-8 expression in human prostate cancer cells by insulin-like growth factor-I and inflammatory cytokines. Growth Horm IGF Res. 2007;17:383–391. doi: 10.1016/j.ghir.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 32.DuBois Raymond N. Cyclooxygenase-2 selective inhibitors and prostate cancer: what is the clinical benefit? J Clin Oncol. 2006;24:2691–2693. doi: 10.1200/JCO.2006.05.9709. [DOI] [PubMed] [Google Scholar]
- 33.Richardsen E., Uglehus R.D., Due J., Busch C., Busund L.T. COX-2 is overexpressed in primary prostate cancer with metastatic potential and may predict survival. A comparison study between COX-2, TGF-b, IL-10 and Ki67. Cancer Epidemiol. 2010;34:316–322. doi: 10.1016/j.canep.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 34.Tkacz V.L., Tohnya T.M., Figg W. Cyclooxygenase-2 and angiogenesis in prostate cancer. Cancer Biol Ther. 2005;4:813–814. doi: 10.4161/cbt.4.8.2089. [DOI] [PubMed] [Google Scholar]
- 35.MacManus C.F., Pettigrew J., Seaton A., Wilson C., Maxwell P.J., Berlingeri S. Interleukin-8 signaling promotes translational regulation of cyclin D in androgen-independent prostate cancer cells. Mol Cancer Res. 2007;5:737–748. doi: 10.1158/1541-7786.MCR-07-0032. [DOI] [PubMed] [Google Scholar]
- 36.Cronauer M.V., Schulz W.A., Burchardt T., Ackermann R., Burchardt M. Inhibition of p53 function diminishes androgen receptor-mediated signaling in prostate cancer cell lines. Oncogene. 2004;23:3541–3549. doi: 10.1038/sj.onc.1207346. [DOI] [PubMed] [Google Scholar]
- 37.Vitale I., Galluzzi L., Castedo M., Kroemer G. Mitotic catastrophe: a mechanism for avoiding genomic instability. Nat Rev Mol Cell Biol. 2011;12:385–392. doi: 10.1038/nrm3115. [DOI] [PubMed] [Google Scholar]
- 38.Fragkos M., Beard P. Mitotic catastrophe occurs in the absence of apoptosis in p53-null cells with a defective G1 checkpoint. PLoS ONE. 2011;6:e22946. doi: 10.1371/journal.pone.0022946. [DOI] [PMC free article] [PubMed] [Google Scholar]