Figure 2.
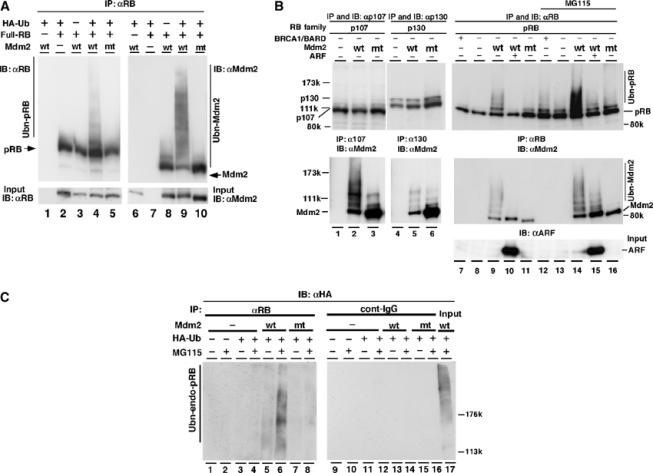
Mdm2 facilitates ubiquitination of pRB in vivo. (A) The full-length human RB expression plasmid pcDNA4-HisMax-hRB (full-RB) was transfected together with pcDNA4-HisMax wild-type (wt) or pcDNA4-HisMax-RING-finger mutant Mdm2C438A (mt) and pCGN-HA-ubiquitin (HA-Ub) into NIH3T3 cells. At 40 h after transfection, cells were incubated with or without 20 μM MG115 for 8 h. The immunoprecipitates obtained with anti-pRB antibody (G3-245) from cell lysates were separated by SDS–7% PAGE and detected by IB with anti-pRB (lanes 1–5) or anti-Mdm2 (lanes 6–10). In all, 3% of the cell lysate for IP was used in an input sample. wt-Mdm2 possessing self-ubiquitination activity promoted ubiquitination of pRB. (B) Full-length RB family expression plasmids of pRB, p107 or p130 were transfected into HEK293 cells with or without the plasmids of Mdm2 (wt or mt), BRCA1-NT (BRCA1) and myc-ARF (ARF). BRCA1-NT, an N-terminal region of BRCA1, is known to retain the ubiquitin ligase activity (Hashizume et al, 2001). To activate BRCA1-NT, BARD1, a cofactor for BRCA1, was coexpressed. Plasmid of HA-Ub was cotransfected in all lanes. The immunoprecipitate obtained with anti-pRB (C-15), anti-p107 or anti-p130 was subjected to IB with anti-pRB (C-15), anti-p107 or anti-p130. Co-immunoprecipitated Mdm2 was monitored with anti-Mdm2 antibodies. In all, 2% of the lysate used for IP was loaded in an input lane and analyzed with anti-ARF antibody. (C) To detect ubiquitination of endogenous pRB, HCT116 cells were transfected with or without the plasmids of HA-Ub or Mdm2 (wt or mt). At 40 h after transfection, cells were incubated with or without 20 μM MG115 for 10 h. The cell lysates were immunoprecipitated (IP) with anti-pRB antibody (G3-245) or control immunoglobulin (cont-IgG) and separated by SDS–6% PAGE. Ubiquitination of endogenous pRB was detected by IB with anti-HA (12CA5) antibody.
