Figure 3.
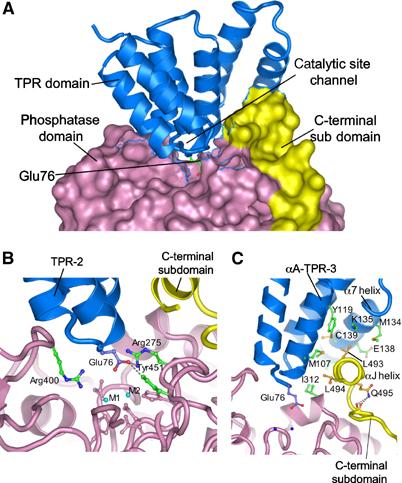
Phosphatase–TPR domain interactions. (A) The phosphatase domain and C-terminal subdomain are represented with a molecular surface, and the TPR domain as ribbon. Intra-TPR turns form a ridge that inserts into the phosphatase domain catalytic channel, with Glu76 of TPR-2 projecting towards the binuclear metal centre. (B) Glu76 of the TPR-2 interacts with Arg275 and Tyr451 at the catalytic site. Metal ions are indicated as M1 and M2 and side chains of metal ion-binding residues are coloured pink. (C) The αJ helix forms hydrophobic contacts with the TPR domain. Detailed interactions involving Leu493 and Leu494 of the αJ helix with TPR-3 and α7 of the TPR domain are shown. The amide side chain of Gln495 donates hydrogen bonds to the main-chain carbonyls of 489 and 490, stabilising the position of the αJ helix.
